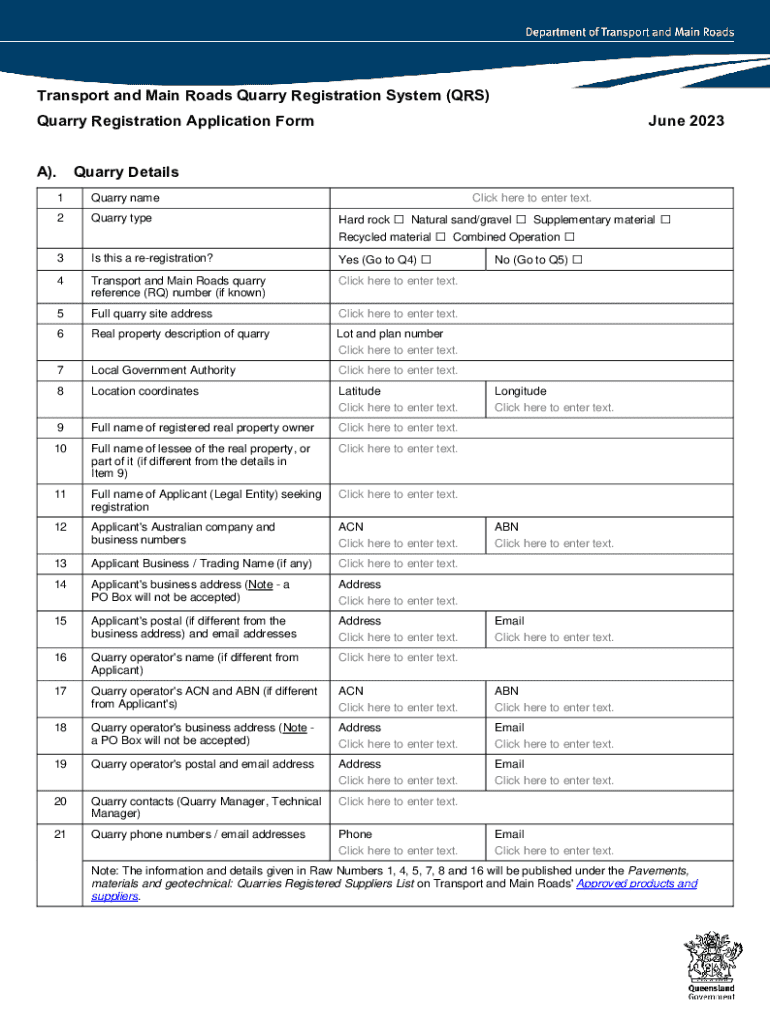
AU QRS Quarry Registration Application Form - Queensland 2023-2025 free printable template
Get, Create, Make and Sign system qrs quarry registration



Editing system qrs quarry registration online
Uncompromising security for your PDF editing and eSignature needs
AU QRS Quarry Registration Application Form - Queensland Form Versions
How to fill out system qrs quarry registration

How to fill out laboratory registration registered suppliers
Who needs laboratory registration registered suppliers?
Understanding the Laboratory Registration Registered Suppliers Form
Understanding laboratory registration
Laboratory registration is a critical process that establishes vendors as credible, reliable suppliers within the laboratory sector. This registration often serves as a gateway to opportunities and offers legitimacy to the suppliers, ensuring that their products meet necessary regulatory standards. In a world where compliance and quality assurance are vital, the importance of laboratory registration cannot be overstated.
Registered suppliers benefit greatly through increased visibility in a competitive market, access to tenders, and the potential for long-term partnerships with laboratories. The registration process generally involves submitting a detailed form that outlines the supplier's credentials, capabilities, and compliance with industry standards.
However, several misconceptions about laboratory registration permeate the industry. For instance, some suppliers assume that registration is only necessary for large organizations, or that it is burdensome and overly complicated. In reality, registration is essential and can be streamlined with the right tools and resources.
Who needs to register as a supplier?
Not every supplier is automatically required to register. Eligibility for laboratory suppliers typically depends on several factors, including the products they offer and the markets in which they operate. Generally, laboratories seek suppliers who provide consumables, instruments, or services that directly impact research, diagnostics, or clinical activities.
Compliance with regulations and industry standards plays a crucial role in determining eligibility. Suppliers of medical devices, chemical reagents, and laboratory equipment must demonstrate adherence to quality control protocols, as well as safety standards, to qualify for registration.
Preparing for the registration process
Prior to submitting the laboratory registration registered suppliers form, it is crucial to prepare by gathering all necessary documentation. Having your company’s credentials organized not only simplifies the application process but also demonstrates professionalism to potential partners.
Key documents typically required for registration include:
Organizing these documents effectively can streamline the process. Use folders to categorize each document and ensure all information is current and accurate to avoid unnecessary delays.
Step-by-step guide to completing the laboratory registration form
Completing the laboratory registration registered suppliers form can seem daunting at first. However, by breaking it down into manageable steps, the process becomes more straightforward. Start by accessing the form on pdfFiller, which provides an efficient way to complete the necessary paperwork.
Once you have the form, pay careful attention to each section. Here are detailed instructions for each part of the form:
Avoid common mistakes such as leaving fields blank or submitting outdated certificates, as these can delay your application. Always review your entries before submission to ensure clarity and correctness. Consider implementing an example scenario of a fictitious supplier correctly completing the form, showcasing the right way to fill out each section.
Digital tools for enhancing your registration experience
Using digital tools can significantly ease the burdens of the registration process. pdfFiller provides a range of features designed to enhance document management, allowing for more efficient workflow during the registration.
Some key features of pdfFiller include:
A cloud-based platform streamlines the entire process, ensuring that you can work on documents from anywhere, making it easier to update information as necessary.
Navigating the submission process
After filling out the registration form, submitting it correctly is the next crucial step. If you choose to submit online, follow the specific guidelines that your registration body outlines. Most platforms, including pdfFiller, offer seamless submission procedures that allow for instant delivery.
Upon submission, you should receive an acknowledgment; keep this for your records. Typically, the review process can take anywhere from a few days to a couple of weeks, depending on the volume of applications received.
Tracking your application status is essential. Regular follow-ups with the regulatory body provide peace of mind, ensuring you remain informed about your application's progress.
Post-registration: maintaining your supplier status
Having successfully registered, the journey does not end. Maintaining your supplier status is equally important, which often includes renewals and updates of your business details.
Regular compliance checks and audits are common expectations from regulatory bodies. Engaging in robust communication allows you to stay abreast of any changes in standards or renewal requirements, ensuring continual compliance.
Resources and tools for registered suppliers
To fully leverage your registration, accessing the right resources and tools is vital. pdfFiller provides a great array of forms and templates that help streamline application processes for suppliers.
Additionally, look into industry-specific guidelines and best practices that can further strengthen your standing as a supplier. Networking within the supplier community could provide insights and further collaboration opportunities, leading to mutual growth.
Frequently asked questions (FAQs)
As with any registration process, FAQs can clarify common concerns. If your application gets rejected, it's essential to review the feedback provided, make necessary adjustments, and resubmit promptly.
To change your registration details, typically, a formal request or application must be filed with the appropriate body. For questions or support regarding the registration process, reaching out to customer support teams or consulting online communities can often yield quick responses.
Getting support and assistance
Recognizing when support is needed can prevent potential issues during the registration process. pdfFiller offers customer support that can assist with the laboratory registration registered suppliers form and provide troubleshooting resources.
Additional resources can also include online forums or communities specific to supplier registration, where shared experiences and advice can prove invaluable. Participate in workshops and training sessions to continually improve your understanding of compliance and registration procedures.
Leveraging partnerships for success
Establishing strong partnerships within the laboratory sector contributes to your success as a registered supplier. Cultivating relationships with laboratories and industry organizations opens doors to opportunities.
Engaging in collaborative projects or research not only boosts visibility but also enhances reputation. pdfFiller can streamline these connections by facilitating communication and sharing of documents between suppliers and laboratories.
Quick links
For those ready to start their registration journey, quick access to forms and resources is vital. pdfFiller provides direct links to download the laboratory registration registered suppliers form, along with additional tools designed to facilitate the process.
Taking advantage of step-by-step video tutorials can also help guide users unfamiliar with online submission or the usage of pdfFiller's features. With these resources, you'll navigate the registration landscape with confidence.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify system qrs quarry registration without leaving Google Drive?
How can I get system qrs quarry registration?
How do I complete system qrs quarry registration online?
What is laboratory registration registered suppliers?
Who is required to file laboratory registration registered suppliers?
How to fill out laboratory registration registered suppliers?
What is the purpose of laboratory registration registered suppliers?
What information must be reported on laboratory registration registered suppliers?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.
















