Get the free Confirmation of Planned Research Activity (full-time)
Get, Create, Make and Sign confirmation of planned research



Editing confirmation of planned research online
Uncompromising security for your PDF editing and eSignature needs
How to fill out confirmation of planned research

How to fill out confirmation of planned research
Who needs confirmation of planned research?
Understanding the Confirmation of Planned Research Form
Understanding the Confirmation of Planned Research Form
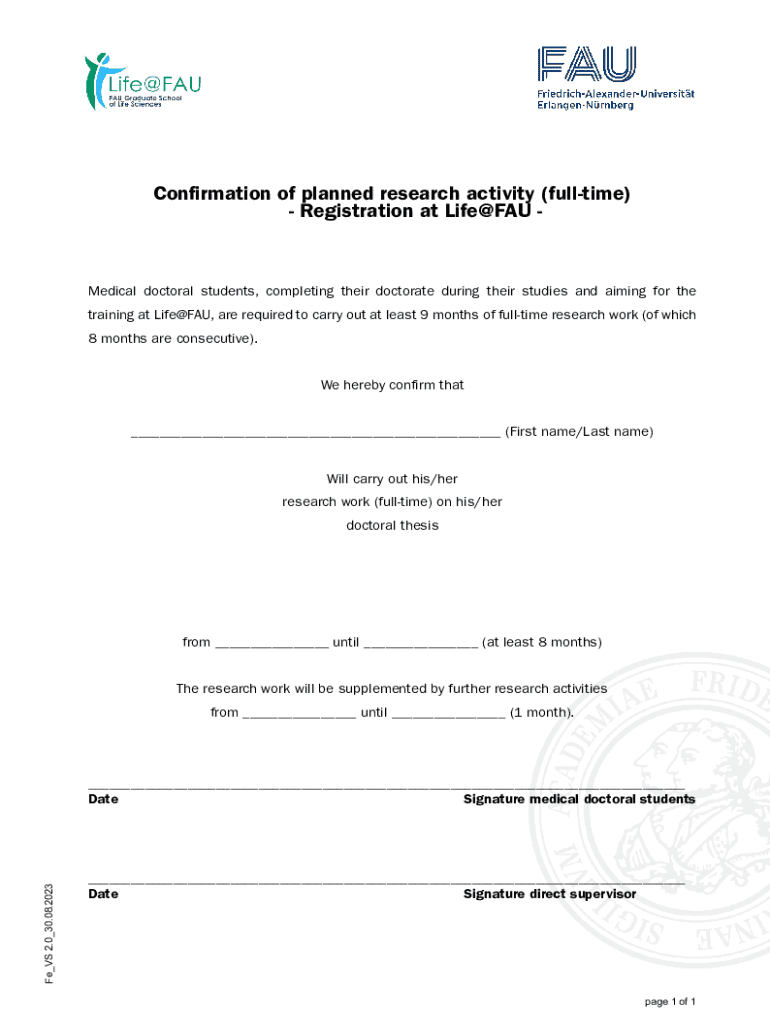
The Confirmation of Planned Research Form is a crucial document in the academic and research community. This form serves as a formal notice of intent to conduct research and encompasses critical information regarding the project. It acts as a foundation for ethical considerations and institutional approvals, thereby ensuring that research is conducted responsibly and within established guidelines.
Its primary purpose is to validate the research plan before data collection begins, effectively serving as a checkpoint in the research process. The importance of this form cannot be overstated; it helps maintain a rigorous standard of accountability, protects the rights of participants, and fosters a culture of ethical research practices in academia.
The process starts with planning, where researchers outline their intended studies. Once drafting is complete, the form needs to be filled out and submitted for validation. After submission, it's reviewed by designated authorities, which may include institutional review boards or advisors, who confirm the feasibility of the study and its compliance with ethical standards.
Key components of a confirmation of planned research form
Completing the confirmation of planned research form requires attention to detail, as it includes essential components necessary for facilitating a clear understanding of your project. Some of these key components include personal details, a concise research title and abstract, as well as well-defined objectives and expected outcomes.
In addition, certain supporting documents may be required, such as excerpts from your literature review, an overview of your methodology, and information about any ethical approvals already obtained. All these components combined present a comprehensive view of your intended research, facilitating informed decisions by reviewers.
Step-by-step guide to completing the confirmation of planned research form
Completing the confirmation of planned research form can seem daunting, but breaking it down into manageable steps simplifies the process. Here's a detailed step-by-step guide to ensure your form is completed accurately.
Common challenges in completing the confirmation of planned research form
While completing the confirmation of planned research form, researchers often face common challenges. These might include submitting incomplete information or misunderstanding the specific requirements of the form, which could lead to delays or rejections.
To overcome these pitfalls, it's crucial to thoroughly read the guidelines provided by your institution. If you encounter difficulties, consider consulting with your advisors who can provide insights and guidance. Utilizing online resources or user communities can also help clarify any uncertainties you may have. Addressing these challenges early on can facilitate smoother completion of your form.
Case studies and best practices
Examining case studies of successfully completed confirmation of planned research forms can provide valuable insights. For instance, a doctoral candidate at a prominent university meticulously filled her form by following departmental guidelines and sought peer feedback, ensuring all necessary components were included and well articulated.
These practices not only increase the chances of approval but also foster an environment of collaboration and academic integrity.
Frequently asked questions (FAQs)
As researchers complete their confirmation of planned research form, they often have common questions that can clarify the process.
Leveraging technology for research form management
In today's research environment, utilizing technology can greatly enhance the management of research forms. Platforms like pdfFiller offer significant benefits for handling the confirmation of planned research forms.
Moreover, pdfFiller provides extensive customer support and resources to help users navigate features effectively, ensuring a smooth form completion experience.
Conclusion and next steps
Completing the confirmation of planned research form is a foundational step toward launching a successful research project. By understanding the requirements, collaborative best practices, and utilizing available technology, researchers can not only streamline their approach but also enhance compliance with ethical standards.
As you progress from confirmation to the actual implementation of your research, maintain organized records of all documentation. Keeping an updated repository of forms and approvals will be invaluable for future research proposals and compliance checks. It's paramount to embrace diligent documentation practices, as this reflects the integrity and rigor of your research endeavors.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify confirmation of planned research without leaving Google Drive?
How do I edit confirmation of planned research in Chrome?
How can I fill out confirmation of planned research on an iOS device?
What is confirmation of planned research?
Who is required to file confirmation of planned research?
How to fill out confirmation of planned research?
What is the purpose of confirmation of planned research?
What information must be reported on confirmation of planned research?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















