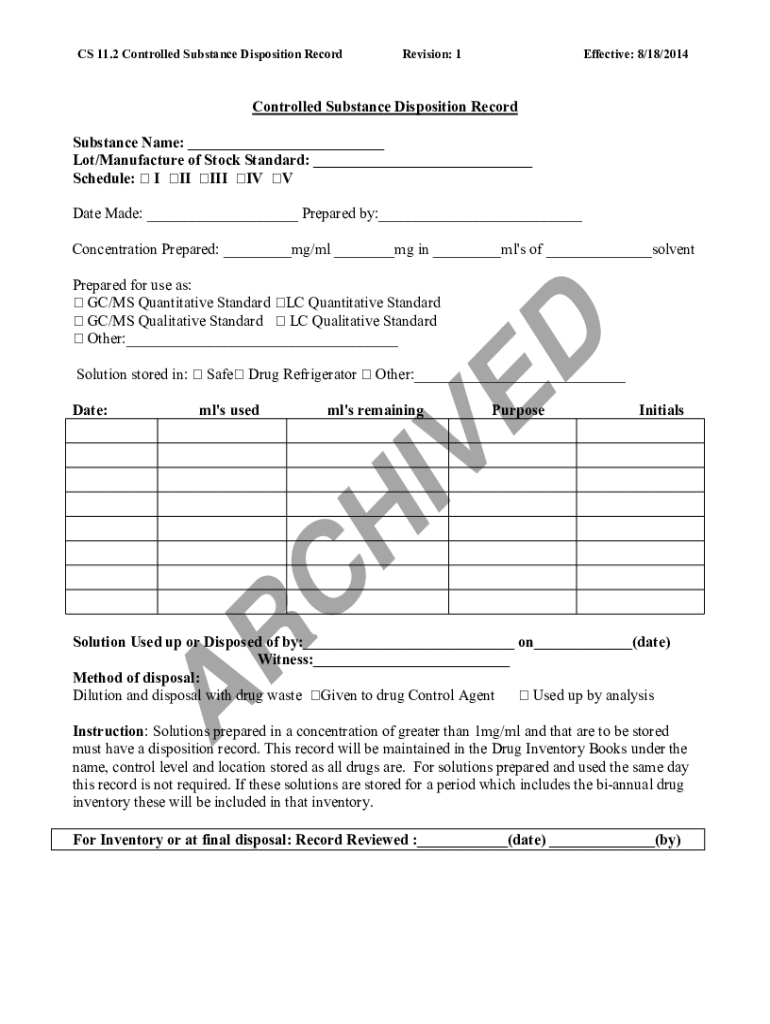
Get the free Cs 11.2 Controlled Substance Disposition Record
Get, Create, Make and Sign cs 112 controlled substance



Editing cs 112 controlled substance online
Uncompromising security for your PDF editing and eSignature needs
How to fill out cs 112 controlled substance

How to fill out cs 112 controlled substance
Who needs cs 112 controlled substance?
A Comprehensive Guide to the CS 112 Controlled Substance Form
Overview of CS 112 Controlled Substance Form
The CS 112 Controlled Substance Form is a critical document in the realm of pharmaceutical and medical practices, designed primarily for the tracking and management of controlled substances. This form ensures that pharmaceutical establishments comply with federal and state regulations, safeguarding both public health and safety. The CS 112 form plays a pivotal role in maintaining an organized inventory, facilitating accountability, and preventing misuse or illegal distribution of controlled substances.
With stricter regulations surrounding controlled substances, the importance of the CS 112 cannot be overstated. It stands as a legal requirement in numerous jurisdictions, and its accurate completion is vital to avoid significant legal repercussions. Ensuring proper management of these substances protects not only healthcare providers but also the patients who depend on safe and effective medication.
Key features of the CS 112 form
The CS 112 form encompasses several key sections that must be accurately filled out to ensure compliance and transparency. Each section provides essential information for both regulatory bodies and healthcare professionals.
The most crucial sections of the CS 112 form include:
Additionally, the form is accessible online and permits various forms of submission, including digital formats that enhance the efficiency and accuracy of data management.
How to fill out the CS 112 form
Filling out the CS 112 form requires careful attention to detail. Understanding how to accurately complete each section is vital for compliant management of controlled substances. The process can be broken down into several steps:
When utilizing digital formats like pdfFiller, there are specific tips for effective submission that can minimize errors and ensure smooth processing.
Editing and managing your CS 112 form
Once the CS 112 form is completed, managing and editing the document is essential for ongoing substance control. Utilizing tools like pdfFiller can significantly streamline this process. Key features include:
Another advantage is cloud-based access, which allows for viewing and editing the form from various devices, facilitating collaboration among team members who may need to contribute to substance management.
eSigning the CS 112 form
Electronic signatures are becoming increasingly important in compliance, especially in forms like the CS 112. The integration of eSigning capabilities through pdfFiller enhances efficiency and ensures the document is legally binding. The process involves:
Incorporating eSigning not only speeds up the process but also adds a layer of security that traditional signatures cannot provide.
Collaboration features with pdfFiller
pdfFiller facilitates team engagement during the CS 112 form completion proces. The platform allows you to invite collaborators to edit and review the document simultaneously, thereby increasing accuracy and ensuring that multiple perspectives are considered. Essential features include:
Effective collaboration mitigates risks associated with incomplete or inaccurate submissions by drawing on the collective expertise of your team.
Compliance and regulatory overview
Maintaining compliance with controlled substance regulations is non-negotiable for healthcare providers. The CS 112 form serves as a tangible record of adherence to these laws, helping to minimize risks associated with non-compliance. Key guidelines include:
The consequences of inaccurate or incomplete submissions can be damaging, stemming from operational flaws to legal disputes. Thus, remaining informed and proactive is key.
Benefits of using pdfFiller for CS 112 form management
Utilizing pdfFiller for managing your CS 112 form presents a range of benefits that enhance workflow efficiency and document accuracy. Some notable advantages include:
The integration of these features enables users to manage essential documentation seamlessly and efficiently, ensuring compliance and operational excellence.
Handling common issues with the CS 112 form
Even with the best intentions, encountering issues while filling out the CS 112 form is not uncommon. Addressing these challenges is crucial for maintaining compliance. Here are common issues you might face:
Approaching common issues proactively can prevent setbacks and ensure adherence to necessary protocols.
Future updates and adaptations of the CS 112 form
As regulations concerning controlled substances frequently change, the CS 112 form is likely to undergo updates. Anticipating these changes is essential for ongoing compliance. Notable future considerations include:
Embracing these anticipated developments will contribute to safer practices surrounding controlled substances, benefiting healthcare providers and patients alike.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit cs 112 controlled substance online?
Can I sign the cs 112 controlled substance electronically in Chrome?
How do I complete cs 112 controlled substance on an Android device?
What is cs 112 controlled substance?
Who is required to file cs 112 controlled substance?
How to fill out cs 112 controlled substance?
What is the purpose of cs 112 controlled substance?
What information must be reported on cs 112 controlled substance?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















