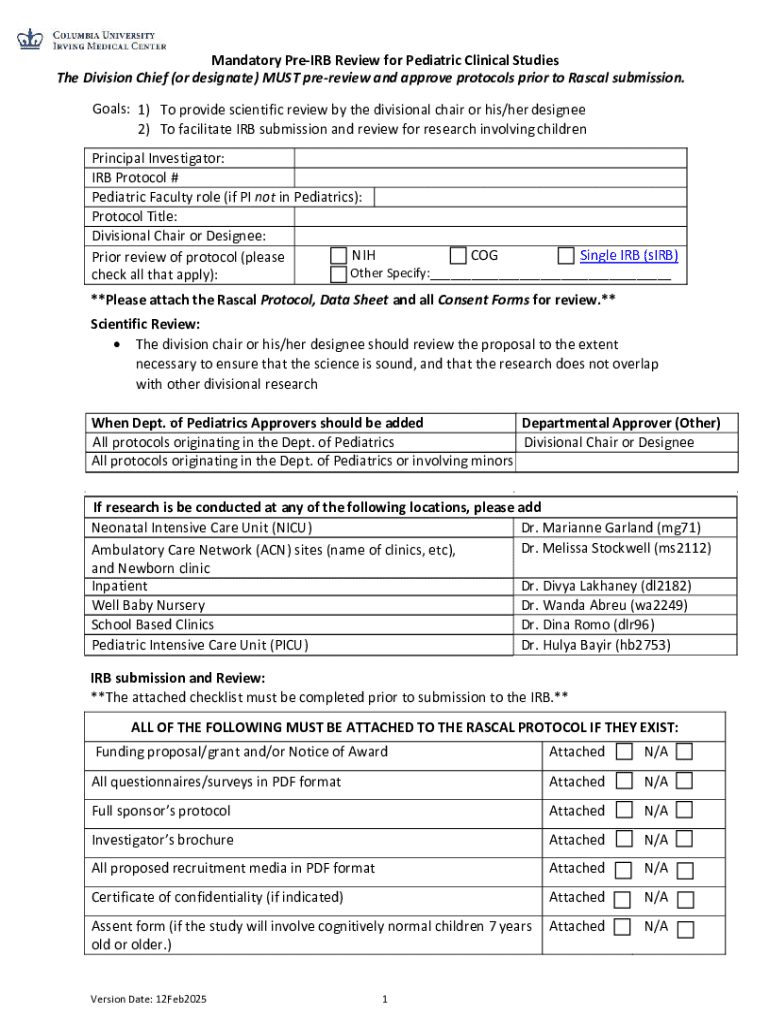
Get the free Mandatory Pre-irb Review for Pediatric Clinical Studies
Get, Create, Make and Sign mandatory pre-irb review for



How to edit mandatory pre-irb review for online
Uncompromising security for your PDF editing and eSignature needs
How to fill out mandatory pre-irb review for

How to fill out mandatory pre-irb review for
Who needs mandatory pre-irb review for?
Mandatory pre-IRB review for form: A comprehensive guide
Understanding mandatory pre-IRB review
An Institutional Review Board (IRB) is tasked with ensuring that research involving human participants is conducted ethically and in accordance with applicable regulations. Mandatory pre-IRB review represents a crucial step before submitting your research proposal for formal IRB evaluation. This early assessment helps to guarantee that your project meets ethical guidelines and is prepared to protect human subjects effectively.
Understanding the significance of pre-IRB review is essential for researchers. It serves as a litmus test for the ethical integrity of a study, ensuring that all scientific inquiries prioritize the safety and dignity of participants. The Human Research Protection Program (HRPP) operates in tandem, providing overarching governance to streamline and safeguard research practices. The HRPP is designed to maintain compliance with ethical standards and federal regulations while also supporting investigator efforts to enhance research quality.
Does your project require pre-IRB review?
Determining whether your research project requires a pre-IRB review hinges on several key factors. The type of research you intend to conduct—be it clinical trials, observational studies, or any other methodological approach—significantly impacts the review requirements. Additionally, the vulnerability of study participants is a critical consideration, as research involving minors, prisoners, or other at-risk groups typically demands a more rigorous review. Lastly, consider the potential impact your research may have on human subjects—greater risks necessitate closer scrutiny.
Specific scenarios, like studies collecting sensitive data or implementing experimental interventions, typically necessitate formal IRB review. Conversely, if your project does not involve human subjects at all—such as data or specimen analysis—it may qualify for non-human subjects research status, which simplifies the review process considerably.
Navigating the IRB review process
Navigating the IRB review process can seem daunting, but breaking it down into manageable steps makes it more straightforward. Here’s a step-by-step guide to aid your progress through the review process:
The IRB offers various types of reviews based on the nature of your research. These include exempt reviews for minimal risk studies, expedited reviews for non-invasive research, and full reviews for high-risk projects requiring extensive scrutiny. Understanding the type of review your project necessitates will help streamline your submission process and set realistic expectations.
Key components of IRB submission documents
Preparing your IRB submission requires meticulous attention to detail, particularly in assembling key components of your documentation. Essential forms and templates may include study protocols outlining the research methodology, consent forms explaining participant rights, and recruitment materials intended to engage potential subjects. Each document must convey clarity and precision, as well-formulated submission materials facilitate smoother reviews and ultimately enhance your project’s credibility.
The importance of clear and concise documentation cannot be overstated, as it significantly impacts the IRB’s comprehension of your study and the overall review timeline.
Understanding review outcomes
Upon completion of the IRB review, your submission may receive various outcomes, each guiding your subsequent actions. An 'approved' status indicates you can proceed with your research, while modifications may be required for projects flagged with certain concerns. Addressing these modifications promptly demonstrates your responsiveness and commitment to ethical research practices. In cases where a project receives a 'disapproved' status, a thorough revision is necessary before resubmitting.
Common reasons for disapproval often stem from inadequate risk management plans, ambiguous study protocols, or poorly defined participant recruitment strategies. Careful attention to detail during the preparation phase can help mitigate these risks.
Post-approval monitoring and compliance
Once you receive IRB approval, your responsibilities as a researcher extend beyond the initial review. Continuous oversight of your project is crucial for ongoing compliance with ethical standards. This involves adherence to any conditions set forth during the approval process, such as periodic progress reports or additional ethical training for team members. Responsiveness to new developments is vital; any unanticipated problems or changes in the study's conduct must be reported to the IRB promptly. Such diligence not only safeguards participant welfare but also aligns your research with institutional and federal regulations.
Monitoring is increasingly essential in studies involving vulnerable populations or high-risk interventions where the landscape may shift, necessitating swift action. Remain vigilant to ensure that your research not only adheres to the approved protocol but also continues to uphold the ethical standards expected by the IRB.
Specific considerations for various research types
Different types of research require tailored ethical considerations. For example, studies involving vulnerable populations, such as minors or prisoners, demand heightened scrutiny and must integrate additional protective measures. Similarly, research involving deceased persons or cadavers poses unique ethical dilemmas requiring specific IRB approval pathways. As researchers increasingly explore quality assurance and quality improvement activities, distinguishing these from traditional research is crucial to determining whether IRB oversight is necessary.
The potential for widespread impacts—whether through direct clinical interventions or broader public health inquiries—necessitates an understanding of ethical frameworks that govern your research design. A well-structured approach not only helps clarify obligations to the IRB but also enriches the scientific contribution of the research.
Interactive tools and resources
In preparing for your mandatory pre-IRB review, leveraging interactive tools and resources can enhance your workflow. Platforms, such as pdfFiller, offer quick links to essential forms and frequently asked questions that demystify the process. Additionally, interactive questionnaires can help determine your need for IRB review—streamlining your pre-application before even entering formal submission pathways.
These tools not only facilitate document creation but also support collaboration among research team members, ensuring everyone is informed and engaged throughout the IRB process.
Latest announcements and updates
Staying informed about recent changes to IRB policies is imperative for researchers aiming to maintain compliance. Federal guidelines and institutional policies can evolve, impacting your project's preparatory stages. Upcoming deadlines for submission, particular reviews, or additional training sessions may highlight important periods when additional attention is warranted. Universities and research institutions may announce workshops or training sessions focused on specific aspects of IRB compliance, providing valuable insights into enhancing your proposals.
Active participation in such sessions not only equips you with critical knowledge but also enables networking with fellow researchers and IRB administrators, fostering a collaborative environment that promotes ethical research practices.
Related topics and further exploration
There are various facets of research compliance to explore, especially if your project involves only data or specimen analysis. Understanding how to request a not human subjects research determination can save time and resources. Additionally, distinguishing between the HRPP and IRB is vital; while the HRPP provides a broader framework for protecting human subjects, the IRB focuses more specifically on reviewing individual studies.
Thoroughly investigating these related topics will enhance your overall understanding of the landscape in which your research operates, developing a holistic approach to ethical research practices. This depth of understanding is crucial as you navigate the mandatory pre-IRB review process and beyond.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute mandatory pre-irb review for online?
How do I complete mandatory pre-irb review for on an iOS device?
How do I fill out mandatory pre-irb review for on an Android device?
What is mandatory pre-irb review for?
Who is required to file mandatory pre-irb review for?
How to fill out mandatory pre-irb review for?
What is the purpose of mandatory pre-irb review for?
What information must be reported on mandatory pre-irb review for?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















