Get the free Kimmtrak (tebentafusp-tebn) Injectable Medication Precertification Request
Get, Create, Make and Sign kimmtrak tebentafusp-tebn injectable medication



Editing kimmtrak tebentafusp-tebn injectable medication online
Uncompromising security for your PDF editing and eSignature needs
How to fill out kimmtrak tebentafusp-tebn injectable medication

How to fill out kimmtrak tebentafusp-tebn injectable medication
Who needs kimmtrak tebentafusp-tebn injectable medication?
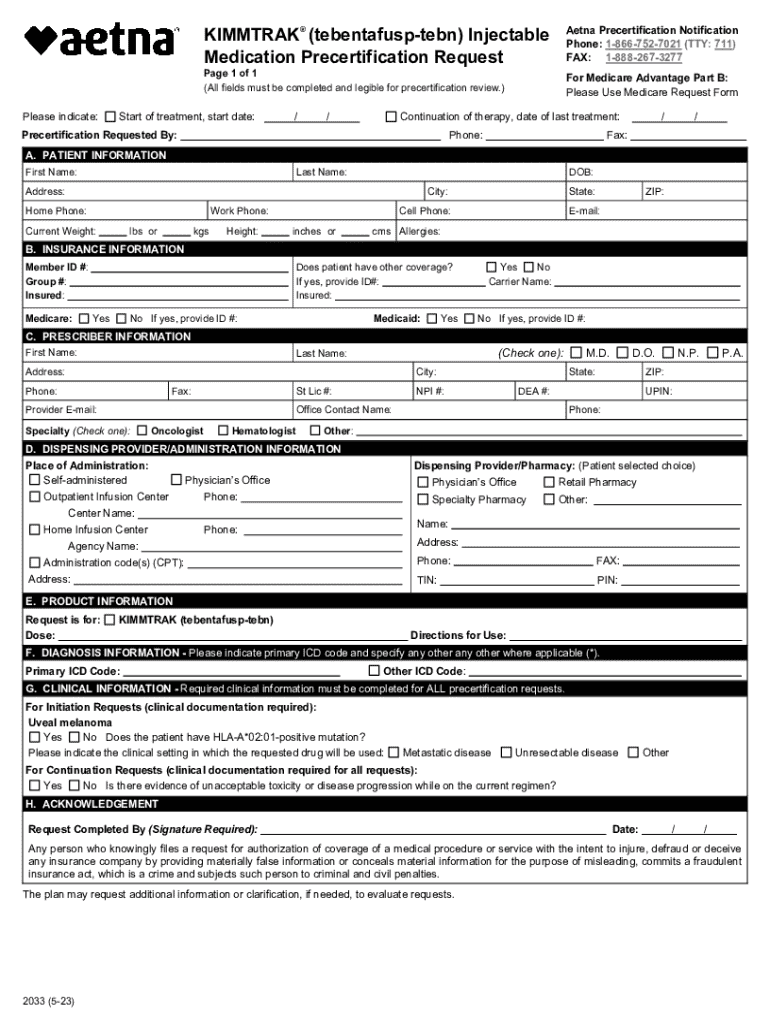
Kimmtrak Tebentafusp-TEBN Injectable Medication Form: A Comprehensive Guide
Overview of Kimmtrak Tebentafusp-TEBN
Kimmtrak, or tebentafusp-TEBN, is a breakthrough injectable medication that targets specific cancer cells, particularly in patients suffering from uveal melanoma, a rare form of eye cancer. The significance of Kimmtrak lies in its innovative mechanism of action, which utilizes the body's immune system to recognize and fight cancerous cells. Approved by the FDA in early 2022, Kimmtrak represents a significant advancement in oncology treatments, offering hope to patients who previously had limited therapeutic options.
This medication is classified as an Immuno-oncology agent, and its therapeutic use aims to inhibit the progression of melanoma, potentially prolonging survival and improving the quality of life for those affected. However, it is essential to be aware of the safety profiles associated with Kimmtrak, including specific restrictions on use for certain populations, which should be discussed thoroughly with healthcare providers.
Reporting side effects
Awareness of side effects is crucial for anyone receiving Kimmtrak. Common side effects include fatigue, rash, fever, and nausea. While these symptoms may be manageable, severe side effects that can arise include severe allergic reactions, liver problems, or eye complications. Patients should remain vigilant and seek immediate medical attention if they experience difficulty breathing, swelling of the face or throat, or persistent abdominal pain.
To ensure patient safety and product efficacy, it's essential to report any side effects experienced. This can be done efficiently through the FDA's MedWatch system, where clinicians and patients can submit reports detailing their experiences. Resources such as the Kimmtrak product label and the manufacturer’s website provide support and guidance on whom to contact for assistance, ensuring any adverse reactions are documented appropriately.
Form completion guidelines
Filling out the Kimmtrak tebentafusp-TEBN injectable medication form accurately is crucial for effective treatment. Incomplete or incorrect information can delay administration or lead to inappropriate dosages. Here’s a step-by-step breakdown of how to fill out the form.
Double-check the completed form for accuracy, and do not hesitate to consult healthcare professionals if any part of the process feels unclear.
Interactive tools for form management
Utilizing electronic tools for managing the Kimmtrak injectable medication form can enhance efficiency and accuracy. pdfFiller provides users with a range of interactive tools to facilitate document handling.
To use these tools effectively, familiarize yourself with the pdfFiller platform, exploring its features to streamline your experience.
Kimmtrak medication indications
Kimmtrak is indicated for patients with HLA-A*0201 positive unresectable or metastatic uveal melanoma. It is crucial to highlight its effectiveness demonstrated in clinical trials, where patients showed significant tumor reduction compared to those receiving the standard of care. Before prescribing Kimmtrak, healthcare providers must evaluate patient eligibility, considering factors such as disease stage and prior treatment history.
Thorough patient evaluation is key to maximizing therapeutic outcomes while adhering to guideline recommendations.
Important safety information
Safety information concerning Kimmtrak is critical for both patients and healthcare providers. Boxed warnings highlight the risks of severe adverse reactions, particularly those involving autoimmune events that may affect multiple organ systems. It is vital to assess the patient’s medical history for contraindications, such as a history of autoimmune diseases or severe allergies.
Implementing mitigation strategies, such as pre-treatment counseling and regular follow-up visits, can greatly reduce potential risks, ensuring patient safety throughout the treatment journey.
Usage instructions for patients and caregivers
When administering Kimmtrak, strict adherence to dosage guidelines is imperative. The recommended dosage should specify administration frequency and volume, typically delivered once per week for the initial treatment, followed by a maintenance schedule tailored to individual response. Patients are advised to report any side effects immediately to their healthcare teams.
Providing caregivers with clear instructions and resources can enhance the overall treatment experience, empowering them to manage the treatment effectively.
Active ingredient breakdown
Tebentafusp-TEBN operates on a unique mechanism that engages the body’s immune system to target cancer cells expressing specific markers. This targeted approach helps to minimize collateral damage to healthy cells, distinguishing it from more conventional therapies that may have broader side effects. Research has demonstrated that this targeted action not only improves patient outcomes but also offers an innovative pathway in cancer treatment.
Understanding the active ingredients in Kimmtrak provides insight into its efficacy, making informed decisions regarding treatment feasible.
Company information and resources
Kimmtrak is manufactured by Immunocore, a leading biotechnology company committed to developing innovative therapies for cancer treatment. Their dedication to patient care is evident through continuous research and support initiatives aimed at improving treatment outcomes for cancer patients globally. Access to customer support services, as well as educational resources about Kimmtrak, is available on the company's website, ensuring that both patients and healthcare professionals have all the necessary information.
For additional educational materials, including FAQs and treatment guidelines, Immunocore provides various resources that can be utilized by patients and healthcare providers alike, further enhancing understanding and optimizing therapeutic strategies.
Quick links for easy navigation
For a seamless experience in managing the Kimmtrak tebentafusp-TEBN injectable medication form, pdfFiller offers a directory that simplifies access to relevant sections and forms. This includes direct links to contact information for professional consultations or support, as well as downloadable resources that detail the usage and administration of Kimmtrak.
By utilizing these resources, users can ensure thorough knowledge and effective management of the Kimmtrak injectable medication form.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete kimmtrak tebentafusp-tebn injectable medication online?
How do I make edits in kimmtrak tebentafusp-tebn injectable medication without leaving Chrome?
Can I create an eSignature for the kimmtrak tebentafusp-tebn injectable medication in Gmail?
What is kimmtrak tebentafusp-tebn injectable medication?
Who is required to file kimmtrak tebentafusp-tebn injectable medication?
How to fill out kimmtrak tebentafusp-tebn injectable medication?
What is the purpose of kimmtrak tebentafusp-tebn injectable medication?
What information must be reported on kimmtrak tebentafusp-tebn injectable medication?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















