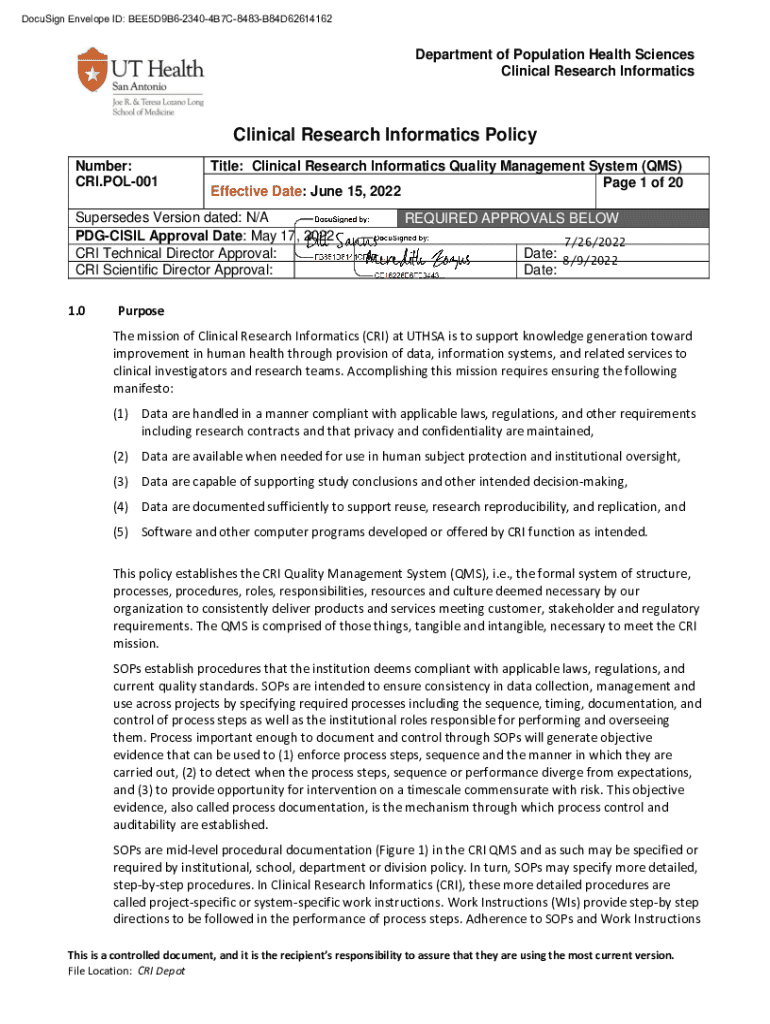
Get the free Clinical Research Informatics Quality Management System (qms)
Get, Create, Make and Sign clinical research informatics quality



How to edit clinical research informatics quality online
Uncompromising security for your PDF editing and eSignature needs
How to fill out clinical research informatics quality

How to fill out clinical research informatics quality
Who needs clinical research informatics quality?
Clinical Research Informatics Quality Form - How-to Guide
Understanding clinical research informatics
Clinical research informatics is a specialized field that focuses on the use of data and technology to improve the quality, safety, and efficiency of clinical research. This domain bridges the gap between health information technology and clinical practices, allowing researchers to make data-driven decisions. With the growing complexity of clinical trials, it becomes imperative to ensure that the collected data maintain high standards of quality.
Quality in clinical research is paramount; it directly influences the credibility and reproducibility of research findings. High-quality informatics means accurate data collection, proper management, and adherence to regulatory standards, which ultimately lead to better patient outcomes and trust in the research process.
Overview of clinical research informatics quality form
The Clinical Research Informatics Quality Form serves as a foundational tool to assess and ensure the quality of clinical research data. Its main purpose is to provide a standardized method for evaluating different aspects of quality throughout the research process. By regularly utilizing this form, research teams can identify gaps, track improvements, and maintain compliance with regulatory agency standards.
Researchers, clinical trial coordinators, and data managers are the primary users of this form. Incorporating the quality form into research workflows allows for early detection of potential issues, fostering stronger project management. Its use results not only in increased efficiency but also in the assurance that the data derived from clinical trials are of the highest quality possible.
Steps to fill out the clinical research informatics quality form
Filling out the Clinical Research Informatics Quality Form involves several important steps that ensure all necessary information is captured accurately. First, researchers must gather participant information while ensuring strict confidentiality and data protection, complying with regulations such as HIPAA.
The form typically contains various sections including demographics, study protocol information, and data collection methods. Each of these sections should be completed thoroughly to provide a comprehensive overview of the research.
Editing and managing your quality form
After filling out the Clinical Research Informatics Quality Form, you may need to edit or manage the document based on ongoing research developments. Utilizing tools available through pdfFiller can enhance this process significantly by allowing users to modify forms easily without disrupting the original layout.
Version control is another vital aspect; it's essential to keep track of changes made to the document as the study progresses. pdfFiller’s platform allows for collaboration with team members, making real-time updates manageable and ensuring all members stay updated with the latest information.
Ensuring data integrity and compliance
Maintaining data integrity within clinical research is non-negotiable, as inaccuracies can jeopardize both the research process and the validity of findings. Establishing best practices for data quality involves implementing strategies like regular audits and cross-verification of data inputs. Integrating quality metrics directly into research efforts can streamline the evaluation process while ensuring compliance with established norms and regulations.
Compliance considerations must also be kept in mind — whether regarding data privacy laws or ethical standards. Research teams should consistently review both their methods and documentation practices to align with organizational and regulatory requirements.
Tools and resources for effective clinical research informatics
Leveraging the right tools and platforms can significantly enhance the efficiency of clinical research informatics. Recommended software should include data management systems, statistical analysis tools, and electronic signing software like pdfFiller, which provides a cloud-based solution for seamless document management.
pdfFiller not only assures document security through encryption but also offers cloud-based accessibility that allows researchers to work from anywhere. This is especially crucial in today's dynamic research environment. Added functionalities, like interactive tools for data analysis, can further optimize research outputs and streamline workflows.
Real-world applications and case studies
Understanding how the Clinical Research Informatics Quality Form applies in real-world scenarios can provide valuable insights for researchers. For instance, a clinical trial that successfully utilized the quality form showcased improvements in data accuracy, which directly influenced patient recruitment strategies. By continuously updating their practices in response to the ratings received through quality assessments, they were able to enhance overall trial efficiency significantly.
Feedback from researchers who have implemented pdfFiller in their projects indicates marked improvements in collaboration and document management. This case showcases the form's impact on the quality of research outcomes, emphasizing how vital documentation is in achieving reliable results.
Common challenges and solutions
Variability in experience levels and resource availability can pose challenges when completing the Clinical Research Informatics Quality Form. Issues such as misunderstanding the required quality metrics or improper data entry can result in decreased effectiveness of research efforts. To overcome these barriers, organizations should consider organizing regular training sessions and workshops for team members, focusing on best practices and the importance of quality in clinical research.
Utilizing pdfFiller can also simplify processes by providing features that minimize errors, like form validation and easily editable fields. Establishing a culture that prioritizes quality assurance can significantly enhance the data integrity of research projects.
Future trends in clinical research informatics
As clinical research evolves, innovative technologies will play a significant role in shaping quality assurance processes. The integration of AI and machine learning for data analysis and quality control is on the rise, promoting increased efficiency and accuracy. The focus will shift towards developing automated systems capable of real-time monitoring and assessments of study data, significantly reducing the manual effort involved.
The future of clinical research informatics will also see further expansion of collaborative platforms that enhance team connectivity and document management. As remote and hybrid work models expand, solutions like pdfFiller will increasingly become essential tools for research teams, enabling smoother workflows and reliable quality documentation across different environments.
Tips for successfully implementing the clinical research informatics quality form in your research projects
Successfully integrating the Clinical Research Informatics Quality Form into your research projects hinges on developing a structured workflow that includes the involvement of all team members. Creating a clear checklist that outlines each step of the form-filling process can aid researchers in ensuring that no detail is overlooked, thus enhancing overall data quality.
Training team members on the importance of the form's role in achieving quality standards is vital. This ensures that everyone understands their responsibilities and the potential impact on research outcomes. Regular review sessions can also facilitate open communication and encourage suggestions for improvements.
Addendum: Quick reference guide
In this comprehensive guide, we've covered crucial steps, challenges, and beneficial tools for handling the Clinical Research Informatics Quality Form. To succinctly summarize key takeaways, a visual flowchart can be included to illustrate the steps for completing the quality form, alongside a FAQ section to address common queries about its usage and significance.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute clinical research informatics quality online?
How do I fill out the clinical research informatics quality form on my smartphone?
How do I edit clinical research informatics quality on an iOS device?
What is clinical research informatics quality?
Who is required to file clinical research informatics quality?
How to fill out clinical research informatics quality?
What is the purpose of clinical research informatics quality?
What information must be reported on clinical research informatics quality?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















