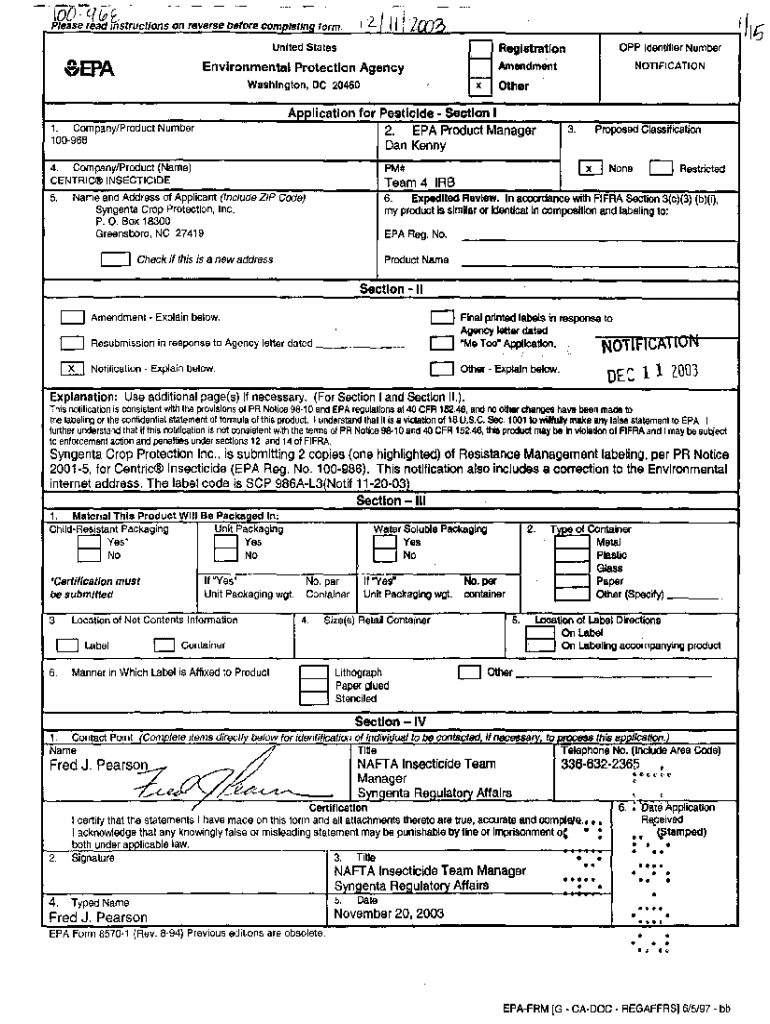
Get the free Epa Notification of Labeling
Get, Create, Make and Sign epa notification of labeling



Editing epa notification of labeling online
Uncompromising security for your PDF editing and eSignature needs
How to fill out epa notification of labeling

How to fill out epa notification of labeling
Who needs epa notification of labeling?
EPA Notification of Labeling Form: A Comprehensive Guide
Overview of EPA notification of labeling form
The EPA Notification of Labeling Form serves as a crucial document for manufacturers and distributors of pesticides and related products. This form is essential for informing the Environmental Protection Agency (EPA) about specific labeling changes or updates. Understanding its purpose is vital for compliance with regulatory requirements and maintaining product integrity.
The primary function of this form is to ensure that all pesticide labels comply with the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) guidelines. The labeling process is not merely bureaucratic; it is a significant aspect of consumer safety and environmental protection, ensuring that products are used correctly and responsibly.
Types of notifications
Labeling notifications
Labeling notifications are required whenever there are changes to the labels of registered pesticide products. This includes alterations in directions for use, warnings, or any claim that might affect the safety or efficacy of the product. Manufacturers must submit these notifications when modifications occur, ensuring that labels reflect the most current and accurate information.
Key requirements for labeling notifications include the submission of complete and accurate label copies, a description of the changes being made, and a statement confirming compliance with EPA standards. Timely submissions prevent potential product labeling violations and enhance the overall safety of pesticide application.
Minor formulation amendments
Minor formulation amendments refer to slight changes in the composition of a pesticide product that do not significantly alter its efficacy or safety profile. Examples include modifications such as changing inert ingredients or adjusting concentrations within permissible limits. These amendments typically require notification but may be processed differently depending on the nature of the change.
Submission criteria for minor formulation amendments include a detailed description of changes, supporting documentation demonstrating that the amendments do not affect overall product performance, and adherence to EPA guidelines. By maintaining transparency through notification, manufacturers contribute to regulatory compliance and consumer trust.
Steps to submit a notification
Step 1: Gather required information
Before submitting the EPA Notification of Labeling Form, it is crucial to gather all necessary information and documentation. This may include existing product labels, data on changes being made, and any relevant studies supporting the modifications. Thorough preparation in this step ensures that submissions are complete and accurate.
Step 2: Completing the notification form
Completing the notification form requires attention to detail. Each field must be filled out accurately, including product name, active ingredients, and specific changes being made. Common pitfalls to avoid include neglecting to update version dates or not providing sufficient detail in the description of modifications.
Step 3: Review process
An internal review process before submission can help catch potential errors or omissions. It's advisable to have team members who are familiar with EPA guidelines evaluate the completed form for compliance. This step can significantly enhance the likelihood of a smooth review and prompt approval by the EPA.
Additional submission considerations
When submitting the EPA Notification of Labeling Form, consider the method of submission. Electronic submissions are preferred for efficiency, as they can streamline the review process. However, paper submissions are also accepted, though they come with specific format requirements, including proper labeling and sealing.
Timeline expectations can vary, but understanding the EPA’s typical review and approval processes helps manage expectations. Typically, electronic submissions may result in quicker review times. Familiarizing yourself with these timelines can aid in planning product launches and updates effectively.
Non-notifications explained
Non-notifications refer to circumstances in which changes can be implemented without formally notifying the EPA. Common examples include label updates that do not significantly alter the product’s use or safety, such as typographical corrections. These instances are important as they can alleviate unnecessary regulatory burden.
Situations when non-notifications may be sufficient include minor changes in marketing language or the addition of non-restrictive statements. Nevertheless, ensuring clarity about what constitutes a non-notification is essential for maintaining compliance and preventing future complications.
Addressing labeling requirements
Essential labeling elements
Every pesticide label must include core elements such as active ingredients, specific usage instructions, and necessary warnings. These components not only facilitate proper application but also ensure that consumers remain informed about potential risks associated with the product.
Bilingual labeling obligations
Regulatory expectations for multilingual labels are increasingly important in diverse markets. Ensuring that labels are available in both English and the predominant language of the target demographic facilitates broader accessibility and compliance with anti-discrimination policies. This practice enhances safety and efficacy among varied user groups.
Recycling and disposal information
Including recycling and disposal information is critical for promoting environmental safety. Labels must provide details about how to properly dispose of or recycle the product packaging. This not only aligns with EPA regulations but also encourages responsible consumer behaviors that help protect the environment.
Handling changes in product chemistry
Types of changes permitted
Changes in product chemistry can be permissible under specific conditions. For instance, minor adjustments related to inert ingredients or categorically non-active components may not necessitate formal notifications, provided they do not alter the toxicological profile of the product.
Documentation required for changes
Maintaining accurate records is vital when handling changes in product chemistry. This ensures that any modifications can be traced back to their original formulations, facilitating compliance with regulatory requirements. Detailed documentation not only aids internal quality assurance but also supports communication with the EPA in case of inquiries.
Special cases in notification
Accelerated review criteria
In certain situations, manufacturers may seek accelerated review criteria to expedite the processing of their notifications. Conditions for applying for expedited processing typically include urgent public health needs or emergency circumstances where rapid action is required. Understanding these criteria can be advantageous for companies facing time-sensitive product challenges.
Grounds for denial of notifications
While submitting the EPA Notification of Labeling Form is a key step, it is equally important to understand common grounds for denial. Issues may arise from incomplete forms, failure to comply with EPA guidelines, or lack of supporting documentation. Addressing these potential pitfalls in advance can greatly improve the chance of acceptance.
Contacts for additional support
EPA contact information
For additional assistance regarding the EPA Notification of Labeling Form, stakeholders can directly contact the EPA through designated regulatory offices. Having this contact information readily available ensures that manufacturers have support for navigating the complexities of submissions.
State and regional contacts
In addition to the federal level, it is important to consider state and regional contacts since various local regulations may affect the submission process. Engaging with local regulatory authorities can provide invaluable insights and prevent compliance issues in different jurisdictions. This proactive approach helps safeguard against unexpected penalties or denials.
Effective communication with regulatory agencies is essential for any manufacturer engaged in the pesticide market. Staying informed about changes in regulations and seeking guidance when necessary can mitigate risks and enhance compliance.
Interactive tools and resources
Templates for notification forms
Using templates for notification forms can significantly reduce the time spent on preparing submissions. pdfFiller offers downloadable resources that streamline the process, ensuring that users can access the most current versions of necessary documents. Such tools enhance efficiency and prevent errors associated with manual preparation.
FAQs and troubleshooting section
A robust FAQs section addresses common challenges that arise during the submission process. By providing clear guidance on typical issues, pdfFiller helps users to troubleshoot problems effectively, fostering a smoother experience throughout the regulatory process.
Webinars and tutorials
Webinars and tutorials are excellent means to enhance understanding of the submission process for the EPA Notification of Labeling Form. These resources can equip users with knowledge about best practices and provide updates on regulatory changes, ultimately contributing to more effective submissions.
Best practices for document management
Efficient document storage is key to managing the EPA Notification of Labeling Form and related submissions. Utilizing platforms like pdfFiller, users can ensure that documents are organized and easily retrievable whenever necessary. This capability contributes to maintaining compliance and improving team collaboration.
Collaboration features within pdfFiller allow teams to work together seamlessly when preparing submissions. This capability not only enhances productivity but also ensures that multiple perspectives are considered when completing forms, leading to more accurate and comprehensive notifications.
The importance of digital signatures for compliance cannot be overstated. Using secured electronic signatures enhances trust and authenticity, ensuring that all parties comply with legal requirements. This capability streamlines the process and helps maintain regulatory standards effectively.
Conclusion: The path to compliance
In summary, navigating the EPA Notification of Labeling Form is essential for manufacturers and distributors in the pesticide market. By understanding the submission process, leveraging tools like pdfFiller, and adhering to best practices, stakeholders can ensure compliance and facilitate smoother interactions with regulatory agencies.
Staying informed about labeling requirements, maintaining detailed records, and utilizing available resources will empower users to manage their documents effectively. Ultimately, the path to compliance is made more accessible through the collaborative capabilities and tools offered by pdfFiller.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my epa notification of labeling in Gmail?
Can I sign the epa notification of labeling electronically in Chrome?
How do I edit epa notification of labeling on an Android device?
What is epa notification of labeling?
Who is required to file epa notification of labeling?
How to fill out epa notification of labeling?
What is the purpose of epa notification of labeling?
What information must be reported on epa notification of labeling?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















