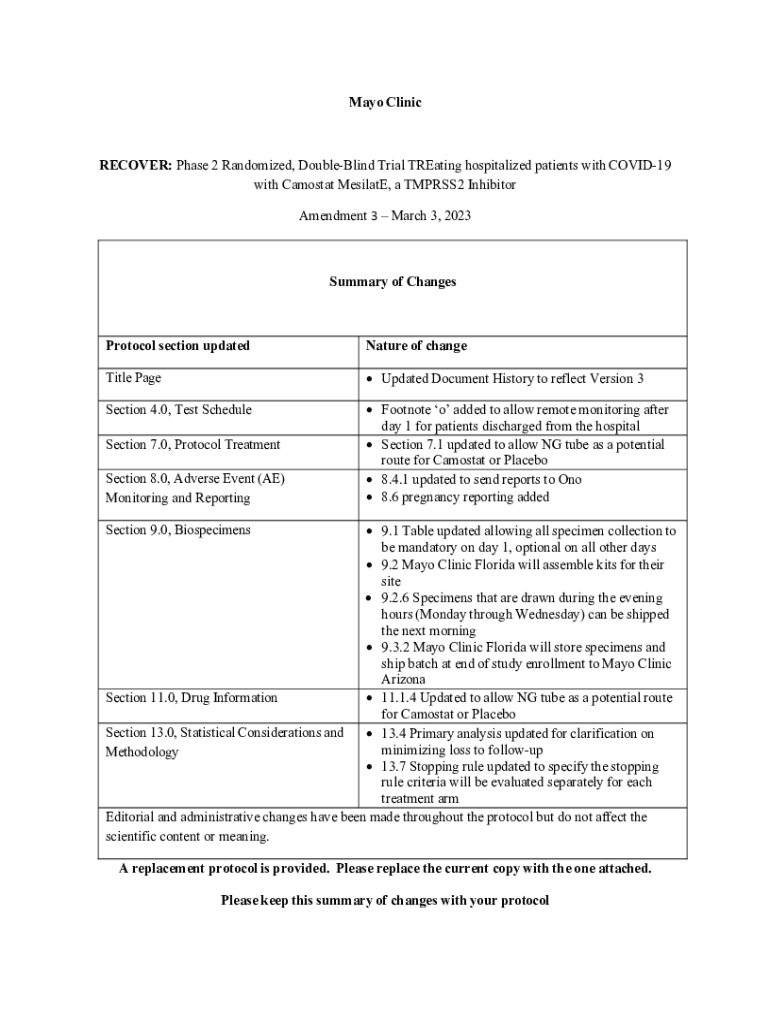
Get the free Recover: Phase 2 Randomized, Double-blind Trial
Get, Create, Make and Sign recover phase 2 randomized



How to edit recover phase 2 randomized online
Uncompromising security for your PDF editing and eSignature needs
How to fill out recover phase 2 randomized

How to fill out recover phase 2 randomized
Who needs recover phase 2 randomized?
Understanding the Recover Phase 2 Randomized Form
Overview of the Recover Phase 2 randomized form
The Recover Phase 2 randomized form is an essential document used to assess cognitive dysfunction following SARS-CoV-2 infection. Designed to gather critical data to evaluate potential therapeutic interventions, this form plays a pivotal role in understanding the cognitive impacts of the virus.
Cognitive dysfunction has emerged as a significant area of concern among COVID-19 survivors, leading to increased interest in targeted recovery strategies. By focusing on individuals who have experienced these impacts, researchers can develop effective interventions to aid recovery in affected populations.
Key features of the form
The Recover Phase 2 randomized form is structured to capture a wide range of necessary information. Its comprehensive design ensures that every relevant detail about a participant's health is considered, starting with demographic data.
Key features of this form include:
The user-friendly interface found on pdfFiller’s platform makes navigating through these sections seamless. Each part of the form is intuitively designed, allowing users to complete it with minimal effort.
How to access the Recover Phase 2 randomized form
To access the Recover Phase 2 randomized form, follow these straightforward instructions on pdfFiller.
This streamlined process allows users to quickly locate and begin filling out the necessary form, saving valuable time during recovery planning.
Filling out the form
Filling out the Recover Phase 2 randomized form accurately is vital for gathering accurate data. Each section has unique requirements that should be carefully considered.
Interactive tools available on pdfFiller, such as smart autofill and data validation checks, are designed to assist users in accurately completing their forms, highlighting the power of technology in modern document management.
Editing the Recover Phase 2 randomized form
After completing the Recover Phase 2 randomized form, you may need to make modifications. pdfFiller offers a suite of editing tools designed for user convenience.
These features are especially helpful for teams managing multiple submissions and ensuring that all contributions are correctly incorporated.
eSigning and submission process
Once the form is filled out and reviewed, it needs to be signed and submitted. pdfFiller facilitates a simple eSigning process to authenticate your submission.
These steps help streamline the submission process, ensuring efficiency and clarity in documentation.
Managing your forms
Effective management of submitted forms is critical, especially in a clinical research context. Here are some strategies to keep track of your documents.
This systematic organization allows teams to manage multiple submissions effectively and remain compliant with research protocols.
Troubleshooting common issues
Completing the Recover Phase 2 randomized form can come with challenges. However, being aware of common pitfalls can minimize errors.
Following these guidelines can significantly reduce the frustration often associated with form completion, enhancing user experience on the platform.
Real-life applications of the Recover Phase 2 randomized form
The real-world impact of the Recover Phase 2 randomized form can be demonstrated through user experiences and outcomes. Many participants have shared success stories regarding their interactions with the form and subsequent therapeutic interventions.
These narratives highlight the necessity and effectiveness of the form in supporting ongoing research and providing valuable insights into patient recovery.
Additional insights and tools
Not only does the Recover Phase 2 randomized form serve a critical function, but pdfFiller also offers additional resources and forms relevant to cognitive assessments and health tracking.
Utilizing these additional features enhances the overall experience and maximizes the utility gained from the pdfFiller platform, ensuring users remain engaged and informed.
Conclusion
The Recover Phase 2 randomized form is a significant resource for both researchers and participants involved in studying cognitive dysfunction associated with SARS-CoV-2. By understanding its features, accessing it efficiently, and accurately completing it, users can contribute valuable data that supports crucial recovery interventions. As part of pdfFiller’s broader offerings, this form exemplifies the platform's commitment to empowering users in document management and collaboration.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send recover phase 2 randomized for eSignature?
How do I fill out recover phase 2 randomized using my mobile device?
How do I edit recover phase 2 randomized on an Android device?
What is recover phase 2 randomized?
Who is required to file recover phase 2 randomized?
How to fill out recover phase 2 randomized?
What is the purpose of recover phase 2 randomized?
What information must be reported on recover phase 2 randomized?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















