Electron configurations and orbital form: A comprehensive guide
Understanding electron configurations
Electron configuration refers to the arrangement of electrons in an atom's orbitals. This configuration is vital in chemistry as it helps explain how atoms interact, form bonds, and dictate an element's behavior. By understanding electron configurations, chemists can predict the types of chemical reactions and the formation of compounds.
Electrons, the subatomic particles responsible for chemical properties, possess distinct characteristics including charge, mass, and spin. Each electron carries a negative charge, has a negligible mass in comparison to protons and neutrons, and exhibits a quantum property known as spin, which affects how they occupy orbitals.
The basic principles behind electron configurations
Three fundamental principles govern the structure of electron configurations: the Aufbau principle, the Pauli exclusion principle, and Hund’s rule. The Aufbau principle states that electrons fill orbitals starting from the lowest energy level to the highest, ensuring maximum stability. For instance, in a simple atom like hydrogen, the electron occupies the 1s orbital first before moving to higher levels, like 2s or 2p.
This principle dictates that no two electrons in the same atom can have identical quantum numbers, ensuring that each electron occupies a unique state.
According to this rule, electrons will occupy empty orbitals singly before pairing up in the same orbital, optimizing stability.
Structure of electron configurations
Electron configurations are structured into shells and subshells that represent energy levels. Shells are designated by principal quantum numbers (n), while subshells include types like s, p, d, and f based on their shape and orientation. Understanding the hierarchy of these orbitals is essential for visualizing how electrons are arranged within an atom.
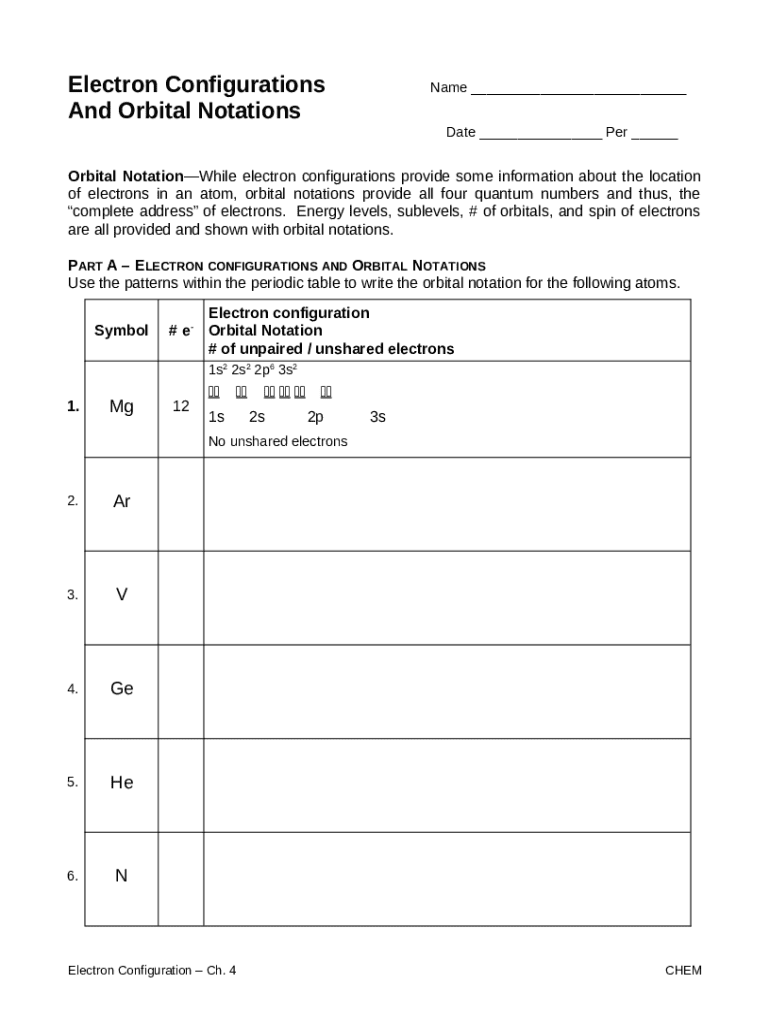
Electron configurations can be represented in various notation systems, most commonly the standard notation, which uses numbers and letters to indicate energy levels and orbital types, and orbital box notation, which uses boxes and arrows to represent electron spins within orbitals. Energy level diagrams visually depict these configurations, allowing for better comprehension of atomic structure.
Writing electron configurations
Writing an electron configuration requires identifying the atomic number of an element, which indicates the total number of electrons. From there, you can systematically apply the aforementioned principles to assign electrons to the appropriate orbitals.
The electron configuration is 1s¹, indicating one electron in the 1s orbital.
The configuration is 1s² 2s² 2p⁴, reflecting its eight electrons across multiple orbitals.
While the expected configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁹, due to stability, the actual configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰.
Orbital diagrams
Orbital diagrams provide a visual representation of electron configurations, illustrating how electrons fill orbitals. They help chemists visualize electron sharing and bonding potential in atoms, especially in more complex elements.
To draw an orbital diagram, one begins by sketching boxes for each orbital type (s, p, d, f) and populates them with arrows representing electrons. Each arrow points up first to indicate spin before pairing occurs in the same orbital. This visual guide assists in understanding electron pairing, and consequently, the behavior of elements.
Special cases in electron configurations
While many elements follow predictable electron configurations, some special cases exist. Transition metals often have irregular configurations due to electron-electron interactions and stability preferences. Elements within the lanthanide and actinide series also exhibit unique behaviors as they fill their f orbitals.
The concept of noble gas configuration simplifies electron configurations by using the nearest previous noble gas as a reference point. For example, the electron configuration of argon (Ar) is often referenced when writing the configurations of elements like chlorine (Cl) to reduce complexity.
Periodic table insights via electron configurations
The periodic table is a systematic arrangement of elements that reflects their electron configurations. Each row and column reveals trends related to atomic properties such as atomic size, electronegativity, and ionization energy, all of which are deeply influenced by the arrangement of electrons.
Generally increases down the groups due to the addition of electron shells and decreases across periods as effective nuclear charge increases.
Trends indicate that electronegativity increases across periods due to increasing nuclear charge, while it decreases down groups.
Ionization energy usually increases across a period and decreases down a group, influenced by electron configuration.
Applications of understanding electron configurations
A comprehensive knowledge of electron configurations is invaluable across various chemistry domains. Understanding how electrons are arranged enhances insight into chemical bonding by revealing how atoms share or transfer electrons to achieve stability and form molecules.
Electron configurations are crucial in predicting reactivity and stability. Elements with similar configurations tend to exhibit comparable properties; thus, discerning these configurations allows chemists to anticipate behavior and reactivity in chemical experiments.
Practice exercises
Engaging with practical exercises reinforces understanding of electron configurations. Fill in the blank configurations provide a hands-on approach to identifying proper electron arrangements. Similarly, orbital diagram challenges test competency in visualizing electron placements.
Practice with prompts that require completing the electron configurations for various elements.
Visually depict the electron arrangement for specified elements, enhancing grasp of orbital filling.
Match element symbols with their corresponding electron configurations to reinforce learning.
Advanced concepts
Exploring the nuances of electron configurations extends into advanced topics, such as molecular electron distribution governed by quantum mechanics. Electron configurations in molecules differ from those in isolated atoms due to the interactions and hybridization effects that also influence chemical bonding.
The Aufbau principle has limitations, and as more electrons are considered, factors like electron-electron repulsion and relativistic effects come into play. The Madelung rule guides the order of filling orbitals but has exceptions, especially within the transition metals and inner transition series, requiring a deeper understanding of each unique configuration.
Key concepts and summary
In summary, electron configurations reveal essential principles that govern atomic behavior, dictating bonding characteristics and elemental properties. Knowledge of the Aufbau principle, Pauli exclusion principle, and Hund’s rule forms the foundation for accurately writing and analyzing configurations.
Recognizing electron configurations not only assists in understanding the periodic table and chemical reactivity but also primes individuals for more advanced studies in quantum chemistry and materials science.
Frequently asked questions (FAQs)
The three fundamental rules include the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
They provide insights into atomic behavior, reactivity, and bonding characteristics.
Noble gases include Helium (He) 1s², Neon (Ne) 1s² 2s² 2p⁶, Argon (Ar) 1s² 2s² 2p⁶ 3s² 3p⁶.
Core electrons are those in the inner shell, while valence electrons are in the outermost shell and involved in bonding.
Unique configurations arise from stability effects, electron interactions, and the need to minimize energy levels.
Engaging tools and resources
Utilizing interactive tools enhances comprehension of electron configurations. Online resources allow users to visualize electron placements effectively, while PDF templates for practice provide valuable exercises to solidify knowledge. pdfFiller’s platform can facilitate collaboration and document management related to chemistry studies, enabling team efficiency.