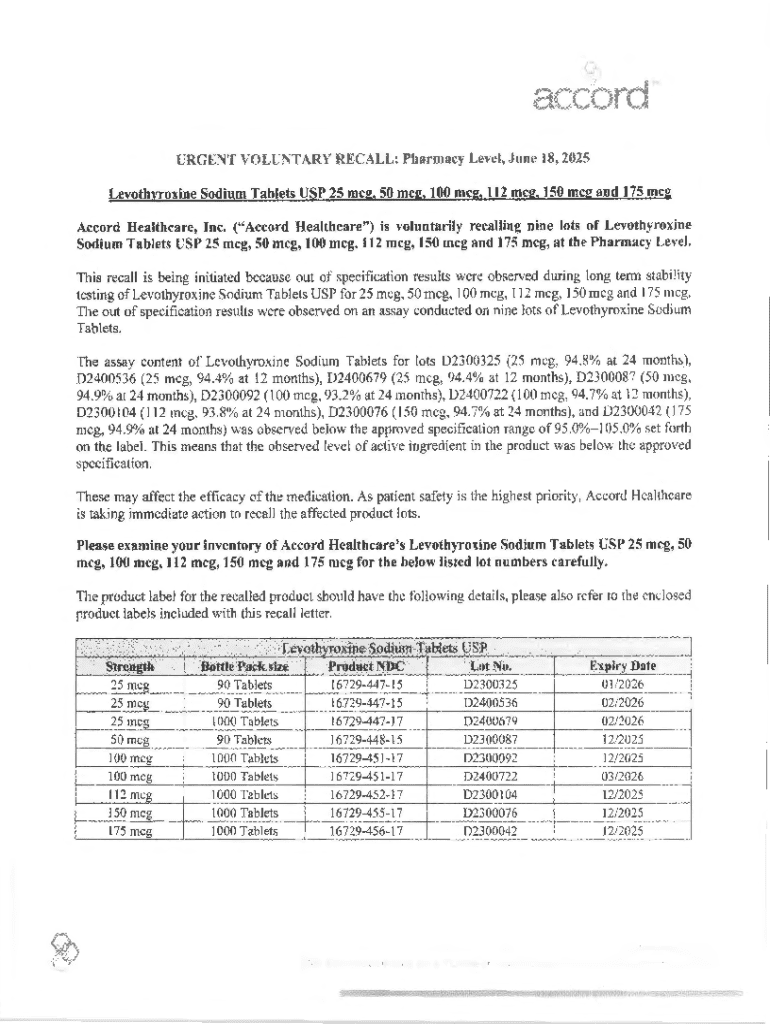
Get the free Drug Recall Report - Community Health Plan of Washington
Get, Create, Make and Sign drug recall report



How to edit drug recall report online
Uncompromising security for your PDF editing and eSignature needs
How to fill out drug recall report

How to fill out drug recall report
Who needs drug recall report?
Understanding the Drug Recall Report Form
Understanding the drug recall reporting process
Drug recalls are critical procedures aimed at protecting public health by removing harmful or potentially harmful products from the market. The drug recall reporting process is the formalized way that manufacturers, healthcare providers, and regulatory bodies communicate about and address issues related to defective or dangerous drugs.
The importance of drug recall reports cannot be overstated. They serve to inform both consumers and healthcare providers about the risks associated with certain medications, thereby facilitating quicker response actions to mitigate health threats.
Key terminology in drug recall reporting includes terms like 'product identification,' which involves the unique identifiers assigned to each drug, and 'recall classifications'—specifically Class I, II, and III, which categorize recalls according to the potential health hazards they pose.
Overview of the drug recall report form
The drug recall report form is a crucial tool in the recall process. Its primary purpose lies in documenting the details surrounding a drug recall and facilitating communication with regulatory authorities such as the FDA. This ensures that all involved parties are informed and that actions taken to manage the recall adhere to regulatory standards.
Components of the drug recall report form typically include vital information about the drug product itself, such as its name, National Drug Code (NDC), and lot number as well as details about the manufacturer and the specific reason for the recall. This structured approach ensures that all critical information is captured succinctly.
Step-by-step guide to filling out the drug recall report form
Before filling out the drug recall report form, it’s essential to gather all necessary documentation to expedite the process. Verify all recall details and product information thoroughly, ensuring that you have accurate and complete information at your disposal.
Ensuring accuracy and completeness is paramount when submitting the report. Cross-check with internal records and be diligent in reviewing information to avoid common errors that could hinder the effectiveness of the recall.
Submitting the drug recall report
Once you’ve filled out the drug recall report form, the next step is submission. Submission can be conducted through various modes, which encompasses electronic submissions through regulatory portals or paper submissions delivered directly to relevant authorities.
After submission, it’s important to confirm receipt and track the submission status. Stay proactive in following up to provide any additional information required and to report on actions taken post-recall submission, ensuring transparency throughout the process.
Best practices for managing drug recalls
Effective management of drug recalls involves creating an internal response team dedicated to managing recalls. This team should have clearly defined roles and responsibilities, ensuring that each member knows their tasks and can act quickly when a recall issue arises.
This approach not only helps in ensuring compliance with regulatory standards but also promotes a safer environment for consumers.
Exploring resources for drug recall management
Accessing regulatory guidelines is crucial for staying compliant with recall standards. Engaging with regulatory bodies such as the FDA provides insights into current regulations and recalls, helping to keep your practice aligned with industry standards.
Sample templates from pdfFiller can be particularly valuable as they save time and ensure that all necessary information is included in a compliant manner, allowing users to focus more on the management of the recall process itself.
Frequently asked questions (FAQs)
When it comes to drug recalls, individuals often have several questions surrounding the reporting process. Key queries include what specific information should be included in a drug recall report and who is ultimately responsible for submitting these reports.
These FAQs emphasize the importance of diligence in the recall reporting process and the need for timely communication and action.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my drug recall report directly from Gmail?
How do I fill out drug recall report using my mobile device?
How do I complete drug recall report on an Android device?
What is drug recall report?
Who is required to file drug recall report?
How to fill out drug recall report?
What is the purpose of drug recall report?
What information must be reported on drug recall report?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















