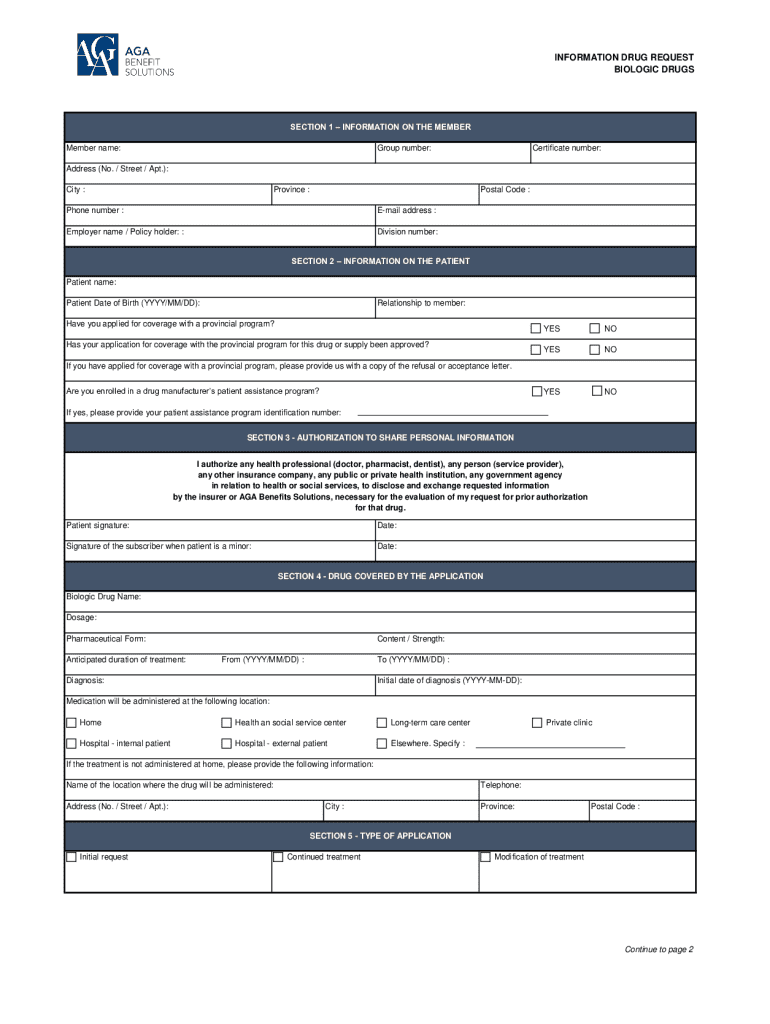
Get the free BIOLOGIC DRUGS
Get, Create, Make and Sign biologic drugs



How to edit biologic drugs online
Uncompromising security for your PDF editing and eSignature needs
How to fill out biologic drugs

How to fill out biologic drugs
Who needs biologic drugs?
Biologic Drugs Form: A Comprehensive Guide
Understanding biologic drugs
Biologic drugs, also known as biologics, are a category of pharmaceuticals that are derived from living organisms. These medications can be made from a variety of sources, including human, animal, or microorganism cells. Unlike traditional drugs, which are typically synthesized through chemical processes, biologics are larger, more complex molecules and can include proteins, monoclonal antibodies, and nucleic acids.
Common therapeutic areas where biologic drugs are utilized include autoimmune diseases, cancers, and various types of inflammation. Their targeted mechanisms allow them to engage with specific pathways and processes involved in disease, offering treatment options where traditional therapies may struggle.
The role of biologic drugs
Biologic drugs play a vital role in modern medicine, particularly due to their ability to harness the body's immune system to fight diseases. They often work by targeting specific interactions within the immune system or by inhibiting certain cellular processes that nurture disease progression. This pinpoint accuracy can yield higher efficacy and potentially fewer side effects compared to conventional drugs.
However, safety considerations cannot be overlooked. The unique mechanisms and complex nature of biologics necessitate diligent monitoring and evaluation in clinical settings to ensure patient safety. The advent of biologics has presented both opportunities and challenges in healthcare, reinforcing the need for healthcare professionals to stay updated with evolving treatment protocols.
Types and classifications of biologic drugs
Biologic drugs can be classified into several major categories based on their composition and therapeutic use. Monoclonal antibodies, for instance, are designed to bind to specific antigens on cancer cells, making them essential in oncology. Vaccines, another form, trigger the immune response and provide immunity against specific infections. Moreover, advancements in cell and gene therapies represent some of the most exciting innovations in the field.
Proteins and peptides, which may function as hormones or enzymes, also fit within the biologics classification, offering tailored treatments for various metabolic disturbances and conditions. Understanding these classifications is essential as they dictate not only therapeutic implications but also how these drugs are developed and administered.
Biosimilars: Understanding the difference
Biosimilars are essentially copies of biologic drugs that have already been approved. They share a high degree of similarity to the reference product but may differ in certain properties due to the inherent variability of biological systems. The FDA has established guidelines for the development and approval of biosimilars to ensure that they meet specific standards of safety and efficacy.
Utilization of biosimilars can provide cost-effective alternatives to original biologics, increasing patient access to essential therapies. However, challenges remain, including variations in manufacturing processes and the need for further studies to establish the interchangeability of these products with their reference counterparts. Understanding these distinctions is crucial for both healthcare providers and patients navigating treatment options.
The biologic drug development process
The development of biologic drugs involves multiple stages, starting from molecular discovery, followed by preclinical testing, where initial safety and efficacy are evaluated in laboratory settings and animal models. The next critical phase is clinical trials, which are divided into three phases—each progressively involving larger participant groups and comprehensive assessments of drug safety and efficacy.
Once clinical trials demonstrate the drug's potential, developers submit regulatory approval applications to relevant bodies, such as the FDA or EMA. Regulatory agencies scrutinize the data from all phases of development before granting approval, which is crucial for ensuring that new biologics are both safe and effective for use in the general population.
Major regulatory bodies and guidelines
In the United States, the FDA oversees the regulation of biologic drugs, a process requiring strict adherence to established guidelines. Similarly, the European Medicines Agency (EMA) provides regulatory oversight in Europe, focusing on the approval and monitoring of biologics. Global perspectives may vary, but the emphasis on rigorous evaluation and post-marketing surveillance remains consistently critical. Post-marketing surveillance is essential for detecting any long-term safety concerns that may emerge once a biologic is widely used.
Understanding the role and guidelines of these regulatory bodies helps stakeholders ensure compliance and improve the safety and efficacy of biologic therapies. Professionals involved in prescribing and managing biologics should be well-versed in these regulations to navigate the complexities inherent in this evolving space.
Filling out biologic drug forms
The process of completing biologic drug forms is a crucial component in ensuring that patients receive the correct medications in a timely manner. Key documents include prescription forms, prior authorization forms, and insurance claim forms. Each of these documents requires specific information to ensure proper processing and approval, especially for high-cost biologics.
Understanding the essential information required is paramount. This includes patient details, prescriber information, the specific biologic drug being requested, and relevant medical history. Common pitfalls involve inaccuracies or omissions that can result in delays or denials of treatment. By utilizing interactive tools, individuals can manage and track these forms more efficiently.
Managing biologic drug documentation
Maintaining accurate and comprehensive records of biologic drug treatments is vital for effective patient care. This includes documenting treatment histories, monitoring patient responses, and securing consent forms when necessary. The importance of secure storage and transfer of information cannot be overstated, as sensitive data must be protected against unauthorized access.
To efficiently review and update documentation, best practices involve utilizing digital tools that enable easy access, revision, and sharing among healthcare teams. Utilizing platforms that allow for cloud-based storage ensures that documentation can be accessed from anywhere, with real-time updates contributing to better patient management and care continuity.
Utilizing PDF tools for biologic drug forms
The transition to digital documentation in healthcare is increasingly vital, especially when managing biologic drug forms. A cloud-based platform like pdfFiller offers significant advantages, including easy editing, eSigning capabilities, and collaborative functions that help streamline the documentation process. Users can access, edit, and manage documents from various devices, ensuring flexibility and accessibility.
Utilizing PDF tools maximizes efficiency by allowing healthcare providers to complete and share forms with minimal delay. Important features include tracking changes, approving signatures in real-time, and storing documents securely in compliance with healthcare regulations. Leveraging these digital tools can enhance productivity and patient service quality.
Special considerations in using biologics
When utilizing biologic drugs, it is essential to consider insurance coverage and associated costs. Biologics, often significantly more expensive than traditional drugs, may require prior authorizations and negotiations with insurance companies. Understanding the insurance forms related to biologics can streamline patient access to necessary medications, mitigating delays in starting treatment.
Additionally, safety and efficacy monitoring are paramount. Regular health check-ups help observe any potential side effects of biologics, and patients must be informed about reporting adverse effects. Patient registries also play a role in enhancing the overall monitoring of biologic treatments and contribute to improved patient outcomes.
Insights and innovations in biologic drugs
The landscape of biologic drugs is rapidly evolving, with emerging trends centered around personalized medicine and advancements in gene therapy. Tailoring treatments based on individual genetic profiles offers the potential to optimize therapeutic effectiveness and minimize adverse effects, rewarding a more precise approach to patient care.
Moreover, interdisciplinary collaboration among healthcare professionals is paramount in managing care for patients on biologics effectively. This teamwork extends from the prescribers, pharmacists, and nurses to specialists to ensure that each patient's treatment plan is comprehensive and considers all aspects of care. Sharing patient experiences and outcomes fosters advocacy and can lead to improved strategies in treatment approaches.
Conclusion and key takeaways
In conclusion, biologic drugs represent a transformative approach to treating various diseases but come with unique challenges regarding their administration and documentation. Understanding how to effectively manage biologic drug forms, from prescriptions to insurance claims, is crucial for optimizing patient care.
Key takeaways include the importance of accurate filling of biologic drug forms, leveraging tools like pdfFiller for efficient document management, and maintaining ongoing communication with patients about their treatments. By doing so, healthcare providers can enhance service delivery and ensure that patients gain timely access to potentially life-saving therapies.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find biologic drugs?
How do I edit biologic drugs in Chrome?
How can I edit biologic drugs on a smartphone?
What is biologic drugs?
Who is required to file biologic drugs?
How to fill out biologic drugs?
What is the purpose of biologic drugs?
What information must be reported on biologic drugs?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.