Understanding the Medical Devices Deficiencies Reporting Form
Overview of medical devices reporting
Medical device deficiencies refer to problems such as design flaws, manufacturing issues, or performance failures that can compromise the safety and effectiveness of medical devices. These deficiencies can arise from various sources, including production processes, material defects, or even software malfunctions within the device. The significance of reporting these deficiencies cannot be overstated, as such reporting is critical for ensuring patient safety and maintaining the integrity of medical devices on the market.
Reporting deficiencies leverages a systematic approach to identify and rectify issues, minimizing potential risks to patients and healthcare providers. Regulatory bodies like the FDA in the United States have specific requirements governing how deficiencies should be reported and documented. Each report contributes to a broader data set that can shape future regulations and standards, ultimately enhancing the safety and performance of medical devices.
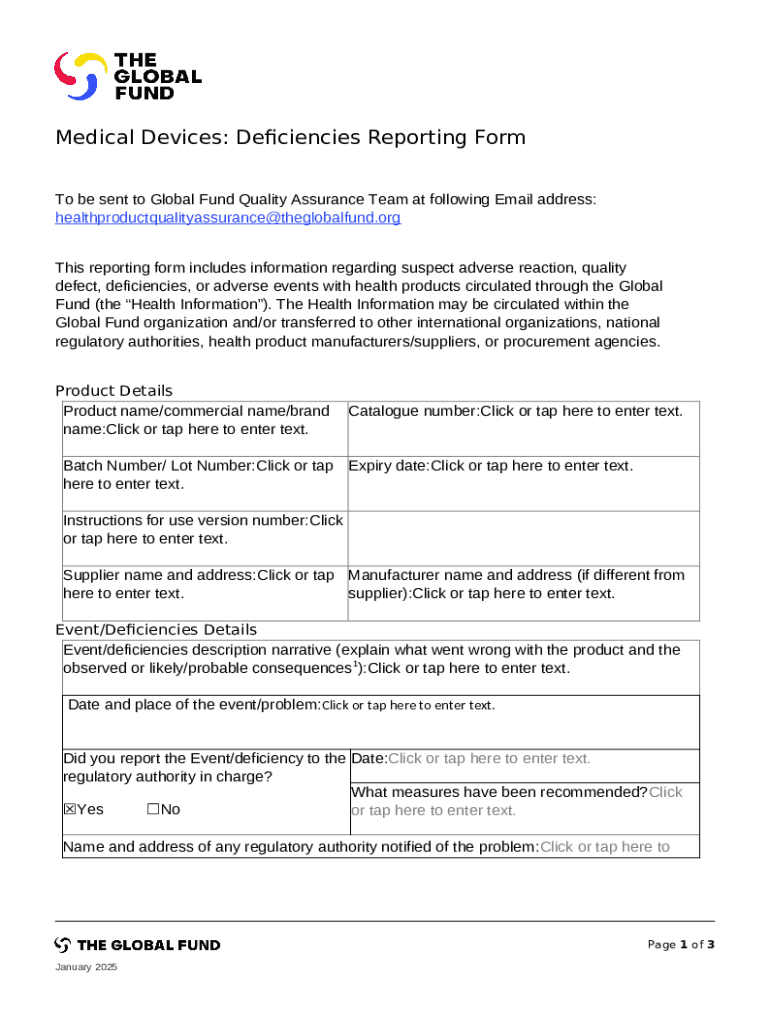
Understanding the medical devices deficiencies reporting form
The medical devices deficiencies reporting form serves a vital purpose; it is designed to capture crucial data surrounding any deficiencies encountered with medical devices. This form acts as a tool for healthcare professionals, manufacturers, and consumers to communicate issues related to medical devices effectively. It not only facilitates the documentation of adverse events but also plays a crucial role in tracking the frequency and severity of these events.
Individuals and entities that need to utilize this form include healthcare providers, device manufacturers, laboratories, and even patients who experience issues with medical devices. The form’s key components include sections for device information, a description of the deficiency, the impact on patients, and details about the reporting party. Thus, accurately filling out this form is an essential part of a coordinated effort to enhance patient safety.
Step-by-step guide to completing the reporting form
Gathering Required Information
Determine if the deficiency pertains to design, manufacturing, or operational failures.
This includes the device's name, model, and lot number, which are essential for accurate reporting.
Detail any occurrences that led to the discovery of the device's deficiency.
Completing the reporting form involves filling out a series of sections in a clear and concise manner. Each section is critical for a comprehensive understanding of the deficiency being reported. Attention to detail during this phase can prevent miscommunication and facilitate prompt action from regulatory bodies.
Include all pertinent details, ensuring that the model and lot numbers are accurate.
Articulate the issue clearly, providing specific examples of how it deviates from expected performance.
Evaluate and note the potential harm or risks posed to patients due to the deficiency.
Ensure the correct date is entered to reflect when the deficiency was detected.
Once you have gathered all the necessary information, carefully filling out the form is crucial. Each section offers an opportunity to detail the situation, so take your time to ensure clarity and accuracy.
Reviewing the Completed Form
Go through a checklist to verify that all sections of the form have been filled out completely.
Avoid vague language, incomplete information, and incorrect dates to reduce delay in processing.
Forms can often be submitted online, via mail, or fax, depending on the governing body.
Be mindful of submission timelines to comply with regulatory requirements.
Keep track of your submission by following up to confirm it was received and inquire about next steps.
Tools for managing your reporting process
Utilizing tools like pdfFiller can significantly enhance your experience with the medical devices deficiencies reporting form. This platform streamlines the documentation process, allowing users to edit, eSign, and collaborate on reports from a cloud-based environment. pdfFiller not only simplifies the management of medical reporting forms, but it also aids in compliance tracking and document storage.
Utilizing pdfFiller for Report Management
Utilizes an intuitive interface that allows easy access to various templates and forms.
The platform enables users to edit forms directly and sign documents electronically, facilitating quicker turnaround.
Encourage teamwork by allowing multiple users to contribute to documents simultaneously.
Accessing and Storing Your Forms
Store forms in the cloud for easy access anytime and from any location.
PdfFiller helps to keep all documents organized, ensuring compliance with future audits.
Adaptations for different audience needs
When navigating the medical devices deficiencies reporting form, it's crucial to tailor your report based on your audience. Individual reporters, such as patients or healthcare providers, may focus on personal experience, emphasizing the immediate impact of the deficiency on their health outcomes. Conversely, corporate teams need to approach the report collaboratively, often requiring different roles and contributions from multiple team members to provide comprehensive documentation.
Take clear notes of your experiences and how the deficiency affected you, focusing on specific events and outcomes.
Implement a review system for documentation that allows team members to input their observations and collaborate on findings.
Understanding regulatory oversight
Regulatory bodies play a crucial role in reviewing medical devices deficiencies reports. They assess the data, identify patterns, and take necessary actions to mitigate risks, which could include recalls or additional requirements for manufacturers. Failure to report deficiencies can lead to severe consequences for both patients and manufacturers, including legal penalties or increased scrutiny from regulators.
Moreover, understanding trends in medical device reporting is essential for ongoing improvements in device safety. By analyzing the data collected, regulatory entities can influence manufacturing standards and enhance compliance measures designed to protect patient health.
Common questions and troubleshooting
As individuals work through the medical devices deficiencies reporting form, numerous questions may arise. Addressing these common inquiries can facilitate smoother completion and submission of the form. Frequently asked questions often include clarifications about specific terms and definitions present in the form, timelines for submission, and what additional documentation may be required to substantiate claims.
Clarifications on terminology, example scenarios, and what constitutes a deficiency.
Identify why a form may be rejected due to incomplete information or misunderstandings regarding reporting techniques.
Provide steps to take when you face obstacles, whether through customer support channels or online resources.
Case studies and examples
Real-life scenarios underscore the importance of reporting deficiencies in medical devices. For instance, an incident involving a faulty defibrillator highlighted how timely reports enabled regulatory agencies to address the issue, leading to swift recalls and heightened awareness around device safety. In contrast, delayed or incomplete reporting can result in injuries, underscores the importance of effective reporting on overall patient safety and compliance.
The positive impact of effective reporting continues to enrich the dialogue between manufacturers, regulatory bodies, and healthcare providers, reinforcing the need for vigilance and proactive communication in the medical device field.
Contact and support information
While filling out the medical devices deficiencies reporting form, assistance can ensure that you complete it correctly and efficiently. Many regulatory bodies and institutions offer resources designed specifically to aid individuals and teams through the reporting process. Online portals frequently contain guides, FAQs, and contact information for support personnel who can answer specific questions regarding the form.
Furthermore, utilizing platforms such as pdfFiller enriches the user experience, providing not just tools for form completion but also customer support to address any hurdles encountered during the reporting process.