Consent Template Form - How-to Guide
Understanding consent forms
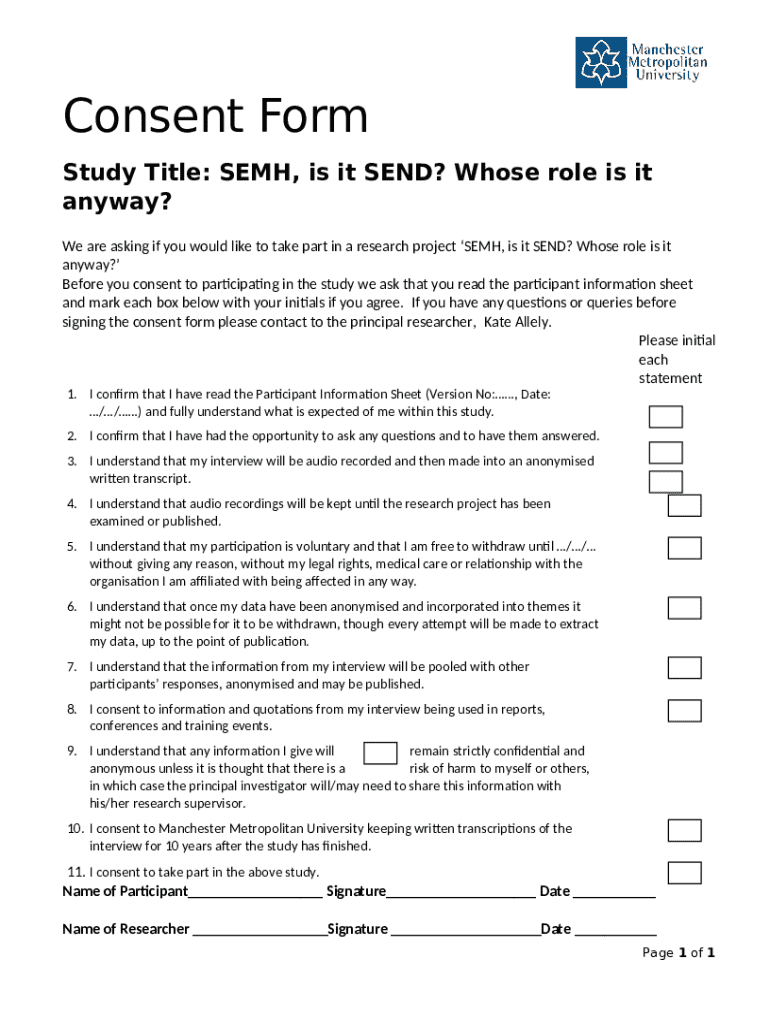
Consent forms are critical documents used in various contexts, primarily to ensure that individuals understand and agree to the procedures or research to which they are consenting. Their importance cannot be overstated: they serve as a record of permission granted by participants, often mitigating legal consequences for researchers and organizations. A well-crafted consent template form communicates essential information clearly and adheres to ethical standards, facilitating trust between parties.
The role of consent forms in documenting permissions is multifaceted. They safeguard rights, promote transparency, and serve as a legal backing in disputes. Such documents are particularly vital in human participant research projects, where informed consent is not just a regulatory requirement but a fundamental ethical practice. By obtaining explicit consent, researchers can ensure that participants acknowledge their rights and the nature of the study.
Protection of participants' rights and autonomy.
Establishment of legal protection for researchers and organizations.
Promotion of ethical research practices through transparency.
Types of consent forms
Consent forms vary based on context and purpose, which means different templates cater to distinct needs. General consent forms are widely applicable and can cover a variety of settings, while specific biomedical procedure consent templates are tailored for use in medical research and clinical practices.
Oral consent templates are another option, useful in scenarios where written consent may not be practical or necessary. In research situations involving minors, child and adolescent assent templates ensure that ethical considerations for involving younger participants are met. Additionally, performance releases are vital in creative fields, allowing artists to document permissions for the use of their performances in various media.
Used for a wide range of applications, generic consent forms ensure broad coverage for various permissions required across different projects.
Designed for detailed, clinical situations, these templates ensure participants are fully informed about medical implications.
Ideal for situations where participants may express consent verbally, ensuring flexibility in collection methods.
Involve guidelines when minors are participants, promoting age-appropriate explanations and parental involvement.
Used in creative industries, allowing performers to grant permission for recording and distribution.
Detailed structure of a consent template form
Crafting an effective consent template form involves incorporating several essential components to ensure clarity and compliance. The introduction should state the purpose of the document, capturing the participant's attention and providing context for what they are agreeing to. Following the introduction, a comprehensive description of the procedure or study is crucial, detailing what participants can expect.
It’s equally important to outline the risks and benefits associated with participation. This section fosters transparency and prepares participants for potential outcomes. Privacy and confidentiality must also be addressed, reassuring participants that their information will be handled securely, in alignment with agency regulations. Providing contact information for any questions or concerns ensures ongoing communication.
Clearly states the purpose and context of the consent.
Offers detailed information on what participation entails.
Communicates potential positives and negatives of participation.
Reassures participants regarding data protection.
Facilitates direct communication for further clarifications.
Step-by-step guide to creating a consent template form
Creating a consent template form involves a systematic approach to ensure all essential elements are included. The first step is to identify the purpose of your consent form; this will guide the content and structure of your document. Next, gather all relevant information regarding the project, including the specific details participants need to know.
Selecting the right template is vital. Accessing resources such as pdfFiller allows for the convenience of choosing pre-designed templates that can be customized. When filling out the form, clarity is key. Use straightforward language and, when applicable, incorporate interactive elements to enhance usability. After drafting the form, a thorough review and editing process is essential to ensure accuracy.
Identify the purpose of your consent form.
Gather relevant information regarding your project.
Choose an appropriate template through pdfFiller.
Fill out the form clearly and comprehensively.
Review and edit the document for accuracy.
Signing and managing consent forms
Effective signing and management of consent forms is integral to maintaining organized documentation in any project. Electronic signing options available through platforms like pdfFiller streamline this process, allowing participants to eSign consent forms conveniently. The step-by-step eSigning process can significantly enhance participant compliance and satisfaction.
Once signed, storing and retrieving consent forms should be systematic. Utilizing document management best practices helps organize forms for easy access while ensuring compliance with legal and ethical standards. This involves categorizing files systematically and implementing security measures to safeguard sensitive information.
Facilitates quick and secure signing processes.
Involves systematic organization to ensure easy access.
Enhances efficiency and compliance when handling documents.
Important considerations when using consent templates
When engaging in the creation and utilization of consent templates, several important considerations come into play. Ethical considerations must always be prioritized, particularly when dealing with vulnerable populations. Recognizing the need for informed consent adapted to suit these groups ensures equitable practices and safeguards their rights.
Jurisdictional variability also plays a crucial role; different areas may have unique legal requirements concerning consent. It is critical to stay informed of any updates or amendments to laws and guidance. Utilizing a template that incorporates current regulations will help in minimizing risk and fostering trust between researchers and participants.
Prioritize protecting participant rights and understanding contexts.
Be aware of local and state differences in consent regulations.
Ensure templates accommodate participants from vulnerable demographics.
Regularly review and revise templates to adhere to current laws.
Interactive tools for consent management on pdfFiller
pdfFiller offers a suite of interactive tools designed to enhance consent management processes. Users can collaborate in real-time on documents, facilitating a seamless review and approval workflow that increases efficiency. Features such as checklists and reference materials can significantly contribute to ensuring all necessary components are included in consent forms.
The platform’s interactive features facilitate easier communication among team members, allowing for immediate feedback and necessary adjustments. These tools not only improve accuracy but also help maintain oversight throughout the entire consent process. Such capabilities ultimately lead to better participant experiences and informed decisions.
Enhances collaborative potential among users.
Facilitates discussion and immediate feedback on documents.
Ensures all necessary elements are included in consent templates.
Common mistakes to avoid
Despite the apparent clarity and purpose of consent template forms, mistakes often occur during their creation and use. One common pitfall is providing incomplete or vague information. Participants require thorough explanations to make well-informed decisions about their involvement, making it vital to detail every aspect of the consent process.
Neglecting legal requirements is another mistake that can have serious ramifications. Researchers and organizations must ensure compliance with all regulatory standards, including obtaining approval from the IRB (Institutional Review Board) when dealing with human participants. Regularly updating templates to reflect current laws and best practices is essential for maintaining their efficacy.
Ensure all necessary details are covered to facilitate informed consent.
Stay informed about regulatory changes pertinent to consent laws.
Review templates periodically to maintain compliance and relevance.
Feedback and support
To maximize the effectiveness of your consent template forms, leveraging feedback from users is invaluable. Organizations should encourage discussions around the usability of their forms, which will help identify areas for improvement. pdfFiller provides dedicated support resources to assist users in navigating their platform efficiently, ensuring that any issues or questions are addressed promptly.
Navigating the pdfFiller support center is straightforward, allowing for quick access to help with document management, form creation, and specific consent template queries. Continuous improvement driven by user feedback not only enhances individual experience but also contributes to overall service quality.
Use the support center and user guides to navigate platform features.
Foster an open dialogue to enhance user experiences.
Access help topics and FAQs for swift assistance.