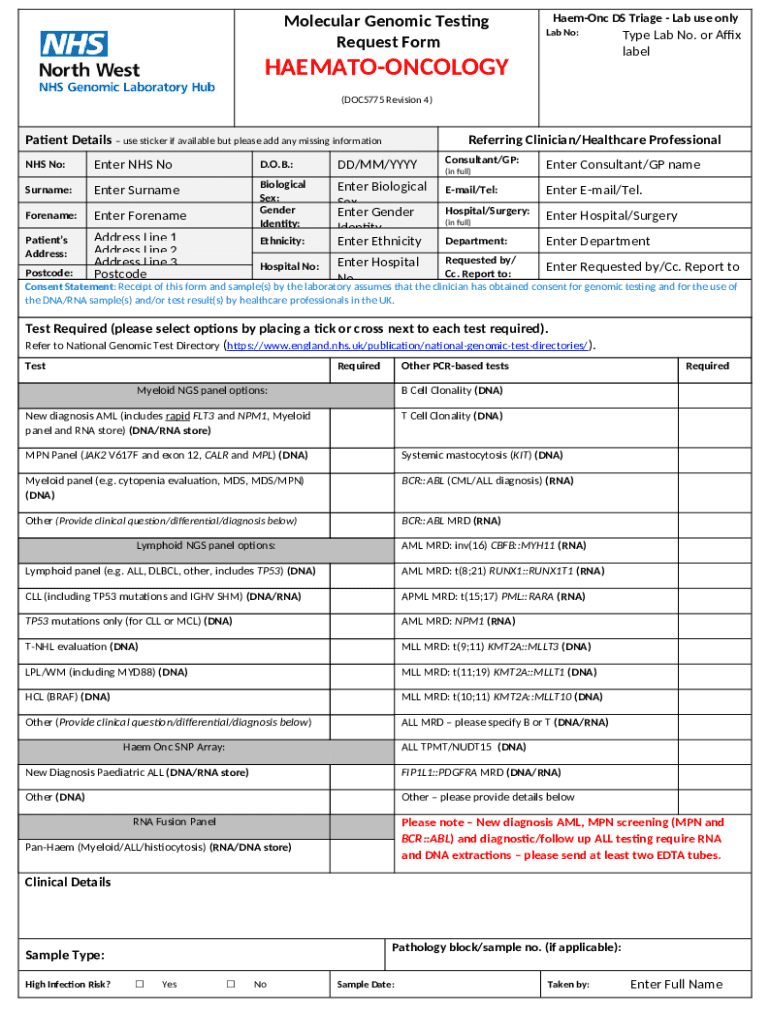
Molecular genomic testing request form: A how-to guide
Understanding molecular genomic testing
Molecular genomic testing refers to a range of techniques that analyze genes, proteins, or metabolites in a patient’s sample—usually blood, tissue, or saliva—to provide crucial information about genetic disorders, hereditary diseases, and even certain tumor types. This type of testing is instrumental in not only diagnosing illnesses but also tailoring treatment strategies specific to the genetic makeup of the disease, particularly in fields like oncology, where genomics plays a critical role in defining treatment pathways.
The importance of molecular genomic testing in modern medicine cannot be overstated. It allows for more personalized medicine, where treatments can be customized based on a patient’s unique genomic profile. For example, in cancer treatment, understanding mutations within solid tumors can guide oncologists in selecting appropriate therapies, potentially improving outcomes. In situations involving rare diseases, genomic testing can provide insights into genetic markers that are critical for accurate diagnosis and treatment, unlocking pathways that were previously difficult to navigate.
Disease diagnosis—facilitates accurate identification of genetic conditions.
Tailored treatment—enables personalized medication based on individual genetic profiles.
Prognostic insights—offers information on disease progression and treatment response.
Molecular genomic testing request form overview
The molecular genomic testing request form serves as an official document enabling patients and healthcare providers to initiate the testing process. Its primary purpose is to capture essential details about the patient and the specific tests being requested, ensuring accurate and efficient processing of the request. Because these tests often involve insurance considerations and medical histories, the form is structured to require detailed and relevant information.
The types of tests covered by the molecular genomic testing request form can include a variety of genomic assays, such as whole exome sequencing, targeted gene panels, and specific mutation tests for solid tumors and blood cancers. Almost anyone can initiate a request—physicians, genetic counselors, or in some cases, the patients themselves. Understanding who can make this request is essential, as it can impact the turnaround time for test results and subsequent patient management.
Preparing to fill out the request form
Before commencing the filling of the molecular genomic testing request form, it’s crucial to gather all necessary information. This includes personal identification details such as name, date of birth, and social security number. Additionally, compiling detailed medical history relevant to the testing—including prior diagnoses, family history of genetic conditions, and any previously conducted genomic tests—will facilitate a smoother process.
Insurance information is another vital aspect to prepare in advance. Ensure you have your insurance policy details handy, as this may help in securing necessary authorizations and coverage for the tests. Familiarity with common terminology found on the form, like specimen type or tumor type, can ease the completion process. A checklist may include items like identification documents, consent forms, and a copy of your referral if required.
Personal identification details including full name and date of birth.
Extensive medical history relevant to the proposed testing.
Comprehensive insurance information with policy numbers and coverage details.
Legal guardianship or consent forms if applicable for minors.
Step-by-step instructions for completing the request form
Filling out the molecular genomic testing request form can be straightforward when approached methodically. The first section typically requires patient information. This section might ask for a comprehensive set of details, including address, contact number, and demographic information. Be diligent about entering accurate information as discrepancies can lead to delays in processing or miscommunication during follow-up.
The next section usually pertains to test selection. It is essential to choose the appropriate tests based on your medical history and physician recommendations. Discussing options with your healthcare provider can ensure that the tests selected align with the clinical context, especially if array testing or specific assays for rare diseases are being considered. In the insurance section, provide accurate coverage details, and if you are uninsured, there may be alternative pathways for financial assistance or self-pay options.
Then, fill in physician information accurately. The form typically requires the physician's name, contact details, and possibly their provider number. The last section highlights the importance of signatures and consent; make sure to review the consent options carefully to fully understand what permissions you are granting. Digital signing is often an option, providing a seamless method for completing this step.
Complete patient information section with accurate contact details.
Select tests carefully based on your discussions with your healthcare provider.
Accurately provide insurance details, exploring options if uninsured.
Input physician’s information, confirming their involvement in the testing process.
Sign and provide consent; ensure understanding of terms before completing.
Submitting the request form
Once the molecular genomic testing request form is accurately completed, the next step involves submission. Depending on your healthcare provider, there may be several methods available for submitting the form. Online portals are a common avenue for electronic submissions, reducing the risk of delays associated with physical mail. Email submission may also be an option if you prefer to keep a digital copy of the request at hand.
If you must submit via physical mail, ensure that you send your completed form to the correct address. To help ensure successful submission, double-check all fields for accuracy and completeness. Following submission, consider tracking procedures by contacting the testing facility or using any provided tracking number. Regular follow-ups help maintain the pace of your testing process and assure any issues are addressed promptly.
Utilize online portals for electronic submission to expedite processing.
Opt for email submission where available, ensuring you maintain a record.
For physical mail, confirm the address and ensure correct postage.
Double-check all fields for accuracy to prevent processing errors.
Establish follow-up protocols to stay informed on the status of your request.
After submission: next steps
After you have submitted your molecular genomic testing request form, there are several steps to anticipate. Firstly, you will receive confirmation of your submission, which may include a tracking number or case reference that allows you to monitor the status of your request. Depending on the laboratory or testing facility, processing timelines can vary; however, clarity around these expectations can often be achieved through direct communication with the provider.
It’s crucial to understand the importance of tracking your request. Many labs offer online tracking capabilities where you can check the progress of your request, ensuring that it is in process. Processing timelines can fluctuate based on the complexity of the test, so maintaining communication with your healthcare provider will be vital throughout this period to discuss expected outcomes and any preliminary results.
Editing and managing your request form
Once submitted, accessing and editing your molecular genomic testing request form, especially if adjustments are needed, is an important skill. Many platforms, such as pdfFiller, provide users with the capability to modify their documents post-submission. If information changes or needs updates—such as new medical history or updated insurance information—having access to a cloud-based platform facilitates this process.
Collaboration with medical providers is also streamlined in this digital age. By sharing access to your request form, physicians or genetic counselors can review changes or necessary updates quickly and make informed decisions about your care. Keeping all your documentation organized in one cloud-based system like pdfFiller ensures no critical updates are missed, and continuity of care remains prioritized.
Use pdfFiller for easy access to your submitted documents and forms.
Update information as necessary to keep records current.
Collaborate digitally with medical providers for efficient care management.
Troubleshooting common issues
Despite thorough preparation and execution, issues can sometimes arise when filling out or submitting a molecular genomic testing request form. Common errors might include incomplete fields, inaccurate details, or omissions of essential documents. A proactive approach would be to review your form multiple times, ensuring all information is accurate and comprehensive before submission.
If your submission is rejected, it is vital to understand the reasons behind this and correct them swiftly. Engage with the testing facility to address any specific concerns they might have. Additionally, most platforms like pdfFiller offer support options, including live chat or customer service, to assist you in navigating any challenges that arise during the submission process.
Review your form multiple times to catch errors before submission.
Reach out to the testing facility for clarifications on rejections.
Utilize customer support options offered by platforms like pdfFiller.
Additional considerations
When utilizing online forms for molecular genomic testing, maintaining privacy and data security is paramount. It's essential to ensure that the platform used for submitting the request form adheres to strict confidentiality protocols. pdfFiller employs advanced encryption technologies to protect sensitive information, allowing users to submit and manage their documents with confidence.
Furthermore, understanding the legal implications surrounding the request form can safeguard both patients and healthcare professionals. Being fully informed of your rights regarding data access and sharing can promote trust in the molecular testing process. As molecular testing continues to play an increasingly significant role in personalized medicine, staying abreast of developments in genomics can empower patients to make informed decisions about their health.
Ensure the platform provides strong privacy and security measures.
Stay informed about legal rights regarding genomic data.
Engage with emerging insights in molecular testing for improved personal healthcare.