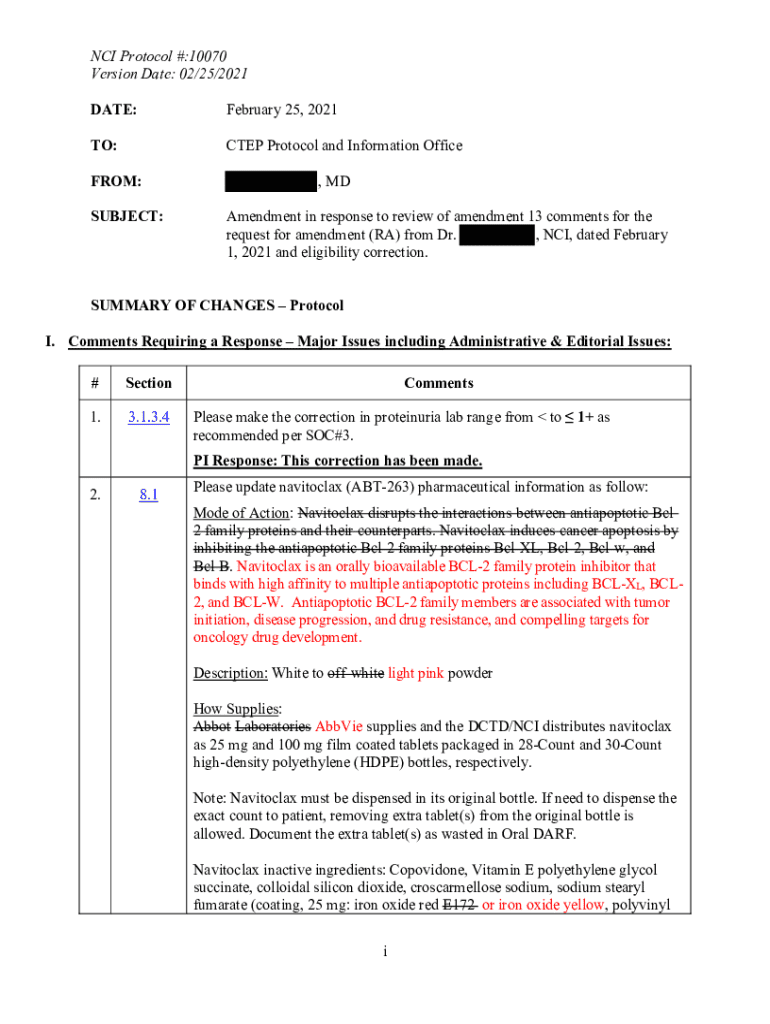
Get the free Nci Protocol #10070
Get, Create, Make and Sign nci protocol 10070



Editing nci protocol 10070 online
Uncompromising security for your PDF editing and eSignature needs
How to fill out nci protocol 10070

How to fill out nci protocol 10070
Who needs nci protocol 10070?
A comprehensive guide to the NCI Protocol 10070 Form
Overview of the NCI Protocol 10070 Form
The NCI Protocol 10070 Form is an essential document designed to facilitate the accurate and efficient collection of data in clinical trials overseen by the National Cancer Institute (NCI). Its primary purpose is to ensure that all relevant information regarding the trial participants and procedures is systematically gathered, documented, and reported. Completing this form accurately not only aids in compliance with regulatory requirements but also enhances communication among research professionals, thereby streamlining the study process.
Historically, the NCI has established specific protocols to standardize data collection across various trials. The Protocol 10070 Form plays a pivotal role in capturing vital details about participant demographics, protocol-specific information, and any adverse events that may occur during the research. This structured approach is pivotal for maintaining the integrity of clinical research trials and ensuring patient safety.
Understanding the components of the form
To effectively utilize the NCI Protocol 10070 Form, it’s crucial to understand its distinct sections and the specific information required. The form typically comprises several critical components:
Before starting to fill out the form, gather all the necessary information, such as participant recruitment strategies, ethical approval documents, and any previous study outcomes that may inform the current trial.
Step-by-step instructions for completing the form
Successfully completing the NCI Protocol 10070 Form requires careful preparation and accurate information. Begin with the following steps:
Once you're prepared, follow this section-by-section guide for filling out the form:
Interactive tools available on pdfFiller
pdfFiller offers an array of interactive tools to enhance the process of completing the NCI Protocol 10070 Form. First, users can easily upload the form onto the platform for editing. With pdfFiller, you can utilize fields, add comments, and make notations to enrich the collaboration process within your team.
One of the most significant advantages of pdfFiller is its real-time eSigning options, which allow for streamlined approvals from all stakeholders involved. Additionally, being a cloud-based platform, pdfFiller enables teams to conveniently access and collaborate on the document from anywhere, enhancing efficiency when gathering input from diverse professionals.
Common mistakes and how to avoid them
Filling out the NCI Protocol 10070 Form can come with challenges, particularly for those new to the process. Some common mistakes include missing or incorrect participant data, inaccurate protocol details, and incomplete adverse event reports. These errors can lead to complications during trial monitoring and compliance checks.
To avoid these pitfalls, consider employing strategies such as:
Following these best practices can vastly improve the quality and reliability of the submitted form.
Collaboration and management features with pdfFiller
pdfFiller’s platform provides valuable collaboration tools that enhance teamwork and efficiency during the form completion process. For example, you can invite team members to contribute directly to the document, offering their expertise and insights. This collaboration can be vital, especially in technical areas like adverse event reporting, which requires precision and accuracy.
Moreover, pdfFiller allows users to track changes and revisions as they occur throughout the document. This feature ensures clarity regarding who contributed what, which can be especially helpful during audits. Furthermore, managing document versions within the platform simplifies storage and retrieval, allowing easy access to previous iterations as required.
After submission: What to expect
Once you've submitted the NCI Protocol 10070 Form, understanding the follow-up process can be beneficial. The submission procedure typically involves sending the completed form to the designated regulatory body or administrator overseeing the trial. After submission, you can expect a confirmation notification to verify that the form has been received.
Be prepared for potential follow-up actions, as review teams may request additional information or clarifications regarding submitted data. It’s essential to remain proactive in tracking the status of your submission- consider maintaining a log or checklist to monitor responses and required actions effectively.
Frequently asked questions (FAQs)
Addressing common queries about the NCI Protocol 10070 Form can significantly assist users in navigating the completion process. One frequently asked question is about the distinction between minor and serious adverse events. Minor events may involve non-life-threatening side effects, while serious events require immediate reporting and further investigation.
Another common inquiry revolves around terminology related to the form. Understanding specific terms such as protocol compliance or data integrity is crucial for accurate form completion. Users should also seek guidance on troubleshooting potential issues encountered during the form-filling process, such as technical errors when uploading files or accessing collaborative tools.
User testimonials and case studies
Numerous users have effectively harnessed the benefits of the NCI Protocol 10070 Form to streamline their clinical trial documentation. Success stories abound, from individual researchers who improved their compliance rates to teams that reduced errors significantly through collaborative efforts using pdfFiller’s features.
Insights from these teams highlight workflows facilitated by pdfFiller, showcasing how streamlined document management led to enhanced data accuracy and timely approvals. These testimonials serve not only as motivation but also as a means to illustrate best practices for others engaging with the NCI Protocol 10070 Form.
Additional features of pdfFiller related to document management
Beyond just the NCI Protocol 10070 Form, pdfFiller provides a suite of advanced document management solutions. This includes secure storage options, robust editing tools for PDFs, and seamless integrations with various platforms, allowing users to centralize their documentation efforts effectively.
To ensure the protection of sensitive patient data, pdfFiller employs strong security measures, including encryption and access controls, which are crucial in the health sector. These features make it a trusted choice for individuals and teams seeking comprehensive document solutions that cater to the complexities of clinical trials and beyond.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find nci protocol 10070?
Can I create an electronic signature for the nci protocol 10070 in Chrome?
How do I fill out nci protocol 10070 on an Android device?
What is nci protocol 10070?
Who is required to file nci protocol 10070?
How to fill out nci protocol 10070?
What is the purpose of nci protocol 10070?
What information must be reported on nci protocol 10070?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















