Get the free Dea Biennial Controlled Substance Inventory Form
Get, Create, Make and Sign dea biennial controlled substance



Editing dea biennial controlled substance online
Uncompromising security for your PDF editing and eSignature needs
How to fill out dea biennial controlled substance

How to fill out dea biennial controlled substance
Who needs dea biennial controlled substance?
Understanding the DEA Biennial Controlled Substance Form
What is the DEA Biennial Controlled Substance Form?
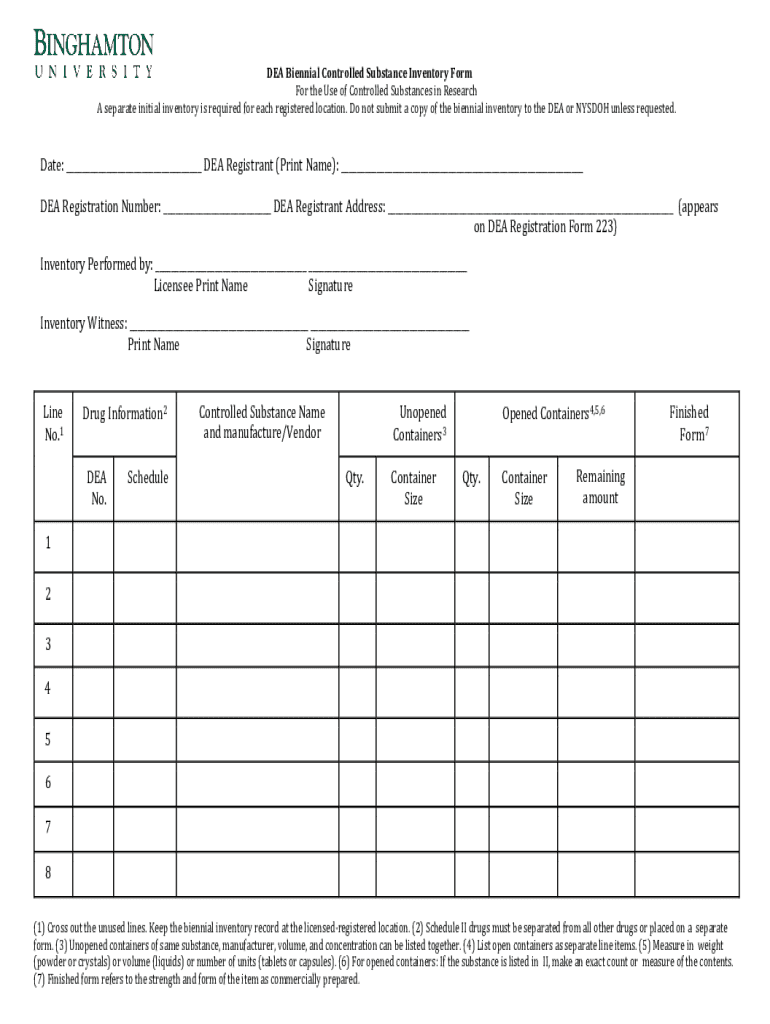
The DEA Biennial Controlled Substance Form is a critical document required by the Drug Enforcement Administration (DEA) in the United States to maintain compliance with the Controlled Substances Act. This form is used to account for the inventory of controlled substances, which includes narcotics and other drugs that have the potential for abuse. The primary purpose of this form is to report an accurate count of all controlled substances held by a registrant, ensuring that these substances are not misused or misappropriated.
Biennial reporting is essential for regulatory oversight, as it helps to prevent drug abuse and promote the safe distribution of controlled substances. Failure to submit this form on the specified schedule can lead to penalties and may jeopardize a registrant's ability to maintain their DEA registration.
Who needs to complete this form?
The completion of the DEA Biennial Controlled Substance Form is mandatory for various individuals and entities that are licensed to handle controlled substances. This includes healthcare professionals such as physicians and dentists, as well as institutions like hospitals and pharmacies. Practitioners who prescribe, administer, or dispense controlled substances are required to complete this form at specified intervals.
Step-by-step guide to completing the DEA Biennial Controlled Substance Form
Completing the DEA Biennial Controlled Substance Form requires careful attention to detail, as missing or incorrect information can lead to compliance issues. The first step is to gather all necessary information, which typically includes your DEA registration number and details about your inventory of controlled substances.
Common mistakes to avoid
When filling out the DEA Biennial Controlled Substance Form, there are several common pitfalls that registrants should avoid. One frequent error is the incorrect calculation of inventory counts, leading to discrepancies between reported and actual quantities. Missing required signatures or not having the appropriate individual sign the form can also result in submission delays.
To prevent these mistakes, it's advisable to double-check all entries or have a designated compliance officer review the form before submission. Utilizing tools available on platforms like pdfFiller can further streamline the process and mitigate errors.
Editing and managing your DEA Biennial Controlled Substance Form with pdfFiller
pdfFiller revolutionizes the way registrants handle their DEA Biennial Controlled Substance Form by offering a cloud-based document management solution. Users can edit, sign, and collaborate on their forms from any location, ensuring they stay compliant while saving time and resources. One significant advantage of pdfFiller is its ease of access; users can store their forms securely online and retrieve them whenever necessary.
Interactive tools for efficient completion
pdfFiller offers interactive tools that simplify the process of completing the DEA Biennial Controlled Substance Form. Users can utilize pre-made templates designed specifically for this form, which include essential fields and validations to ensure accuracy during completion. The interactive nature of these tools allows users to engage with the document in real-time, making corrections or adjustments as needed.
By leveraging these features, registrants can significantly enhance their efficiency in filling out the form. This includes auto-fill options for recurrent data entries and easy navigation between sections, ultimately saving time and minimizing confusion.
Signing and submitting the DEA Biennial Controlled Substance Form
Once the DEA Biennial Controlled Substance Form is completed, it requires a signature for validation. With pdfFiller’s electronic signing functionality, users can sign the document digitally, streamlining the submission process. This not only saves time but also provides legal assurance, as electronic signatures are recognized by most regulatory bodies and hold the same validity as hand-written signatures.
Managing your DEA records post-submission
After submitting the DEA Biennial Controlled Substance Form, it is vital for registrants to keep track of their submissions. Best practices for record-keeping include saving copies of the submitted forms and confirming receipt from the DEA. pdfFiller enables users to efficiently manage these records by allowing them to organize their submissions and related documents digitally, ensuring easy retrieval when needed.
Preparing for future biennial counts
As a registrant, preparing for future biennial counts means adopting a proactive approach to compliance. Schedule periodic audits of your inventory and ensure that all records are up to date. Taking advantage of pdfFiller’s reminder features can facilitate an organized and timely review process.
Establishing a routine to review and update your controlled substance inventory not only ensures compliance but also promotes responsible handling of these substances. Setting aside time for this process can save considerable stress as the biennial reporting date approaches.
Frequently asked questions about the DEA Biennial Controlled Substance Form
Navigating the DEA Biennial Controlled Substance Form can raise questions for registrants at various stages of the process. Common inquiries include clarifications about specific sections of the form, as well as troubleshooting issues that may arise during submission. Understanding the requirements and available supports can help mitigate uncertainties.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I fill out the dea biennial controlled substance form on my smartphone?
How do I edit dea biennial controlled substance on an iOS device?
How do I edit dea biennial controlled substance on an Android device?
What is dea biennial controlled substance?
Who is required to file dea biennial controlled substance?
How to fill out dea biennial controlled substance?
What is the purpose of dea biennial controlled substance?
What information must be reported on dea biennial controlled substance?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















