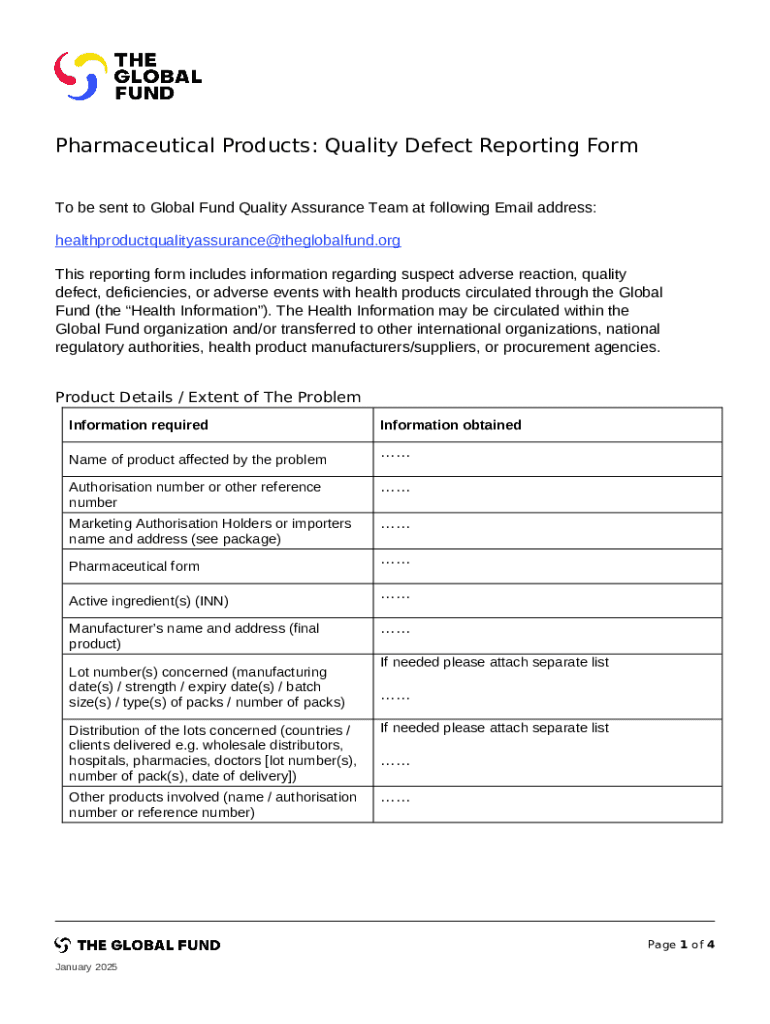
Pharmaceutical Products Quality Defect Form - How-to Guide
Understanding pharmaceutical product quality defects
Pharmaceutical quality defects refer to any deviations from the standards of quality set for medicines. These defects can arise during the manufacturing process, packaging, or distribution and may compromise the safety and efficacy of pharmaceutical products. Recognizing these quality issues is vital for maintaining patient safety, enhancing public trust in the healthcare system, and ensuring compliance with regulatory standards.
Regulatory bodies, such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA), play critical roles in monitoring and enforcing quality assurance in pharmaceuticals. They establish guidelines that pharmaceutical companies must adhere to and oversee the reporting and investigation of quality defects. This ensures that any issues found in supply chains are addressed swiftly.
Importance of reporting quality defects
The act of reporting quality defects is paramount for patient safety and public health. Defective pharmaceuticals can have serious consequences, such as ineffective treatment or, in some cases, severe health risks. Reporting these defects helps regulatory agencies take necessary actions, including product recalls or investigations to prevent future occurrences.
Regulatory agencies rely heavily on data from healthcare professionals, patients, and manufacturers to maintain a robust oversight system. Failing to report quality defects can lead to dangerous situations, misinforming patients and healthcare providers about the safety of a product. Additionally, pharmaceutical companies risk legal repercussions and damage to their reputation when defects go unreported.
Preparing to use the quality defect form
Before filling out a pharmaceutical products quality defect form, gather all essential information needed for accurate completion. This includes details about the product in question, the nature of the defect, and any relevant documentation such as photographs or lab results. Understanding the types of defects, including packaging issues, contamination, and mislabeling, is crucial for effective reporting.
Anyone can report a defect; however, healthcare professionals, patients, and manufacturers are the primary reporters. It's important for those involved in the dispensing or administration of medications to recognize defects and provide complete information to ensure thorough investigations.
Step-by-step guide to filling out the pharmaceutical products quality defect form
Step 1: Accessing the form
To access the quality defect form, visit the pdfFiller platform. Navigate to the designated section for pharmaceutical product reporting, where you can find the specific form. The online platform allows you to conveniently fill out the form from any device, ensuring accessibility.
Step 2: Entering your personal information
Complete the personal information section of the form, including your name, contact details, and role (e.g., patient, healthcare provider). Accuracy is crucial, as it allows for effective communication regarding your report.
Step 3: Describing the quality defect
In this section, provide a comprehensive description of the quality defect. Detail the specific issues observed and include product details such as the name, batch number, and expiration date. Clarifying the circumstances under which the defect was discovered is also essential.
Step 4: Supporting documentation
Attach any relevant documentation that supports your report. This may include photographs that document the defect, lab results, or prior reports related to the product. Use pdfFiller's tools to upload and attach files efficiently.
Step 5: Review and finalize the report
Before submitting, it’s imperative to review your report for accuracy and completeness. Use pdfFiller’s editing tools to make any necessary adjustments to ensure all information is clear and precise.
Step 6: Submitting the quality defect form
Once satisfied with your report, follow the instructions provided on pdfFiller to submit the form online. Be prepared for potential follow-up communications from regulatory agencies, as they may reach out for additional information or clarification.
Keeping track of your submission
After submitting the pharmaceutical products quality defect form, it's important to track your submission's status. pdfFiller enables users to monitor their reports, ensuring that you can stay informed about the follow-up actions taken by regulatory bodies.
Recommended follow-up actions include routinely checking for updates on your submission and remaining in contact with the relevant regulatory agency. This ensures that the reporting process remains transparent and effective.
Engaging with regulatory bodies
Staying engaged with regulatory bodies like the EMA can expedite investigations and issue resolutions. Understanding your role in ongoing communication is vital. When new information becomes available or you receive updates, promptly inform regulatory bodies to assist in their assessments.
Best practices for keeping regulatory agencies informed include maintaining detailed records of communications and updates related to the defect, and being proactive in sharing relevant insights or observations.
Frequently asked questions (FAQs)
If you encounter difficulties while filling out the pharmaceutical products quality defect form, refer to the guidance provided on the pdfFiller platform or seek assistance from a healthcare professional. There are generally no strict deadlines for reporting defects; however, timely submission is encouraged to ensure prompt action.
After you submit the quality defect form, you may receive acknowledgments or requests for more information from regulatory agencies. Being responsive to these communications can greatly assist in ensuring effective investigations.
Lessons learned from reporting cases
Insights gained from successful quality defect reporting experiences often highlight the importance of accuracy and thoroughness in descriptions. There are numerous case studies illustrating how detailed reporting led to decisive actions, such as drug recalls or changes in manufacturing processes that enhanced safety and compliance.
Testimonials from healthcare professionals and patients underscore the role that accurate reporting plays in sustaining product integrity. They emphasize that personal involvement in the reporting process can lead to more vigilant oversight and stronger patient safety protocols.
The role of collaboration in quality assurance
Collaboration among healthcare professionals in reporting defects is essential for maintaining high standards of pharmaceuticals. Creating a culture where team members feel empowered to share observations about product quality can significantly enhance patient safety.
pdfFiller supports team collaboration tools that enable seamless communication and information sharing. Utilizing these tools can foster a more proactive approach to quality assurance and facilitate collective reporting efforts.
Next steps for stakeholders
Pharmaceutical companies must act decisively upon receiving a defect report. This may include issuing recalls, conducting internal investigations, and implementing corrective actions to prevent future defects. Establishing efficient communication channels with regulatory bodies is also vital for transparent reporting.
For stakeholders, continuous education on quality assurance is crucial. Staying informed about evolving regulations and best practices in the pharmaceutical industry can help maintain compliance and safeguard consumer health.