Get the free Quality System Manual
Get, Create, Make and Sign quality system manual



How to edit quality system manual online
Uncompromising security for your PDF editing and eSignature needs
How to fill out quality system manual

How to fill out quality system manual
Who needs quality system manual?
Quality system manual form: A comprehensive guide
Understanding quality system manual forms
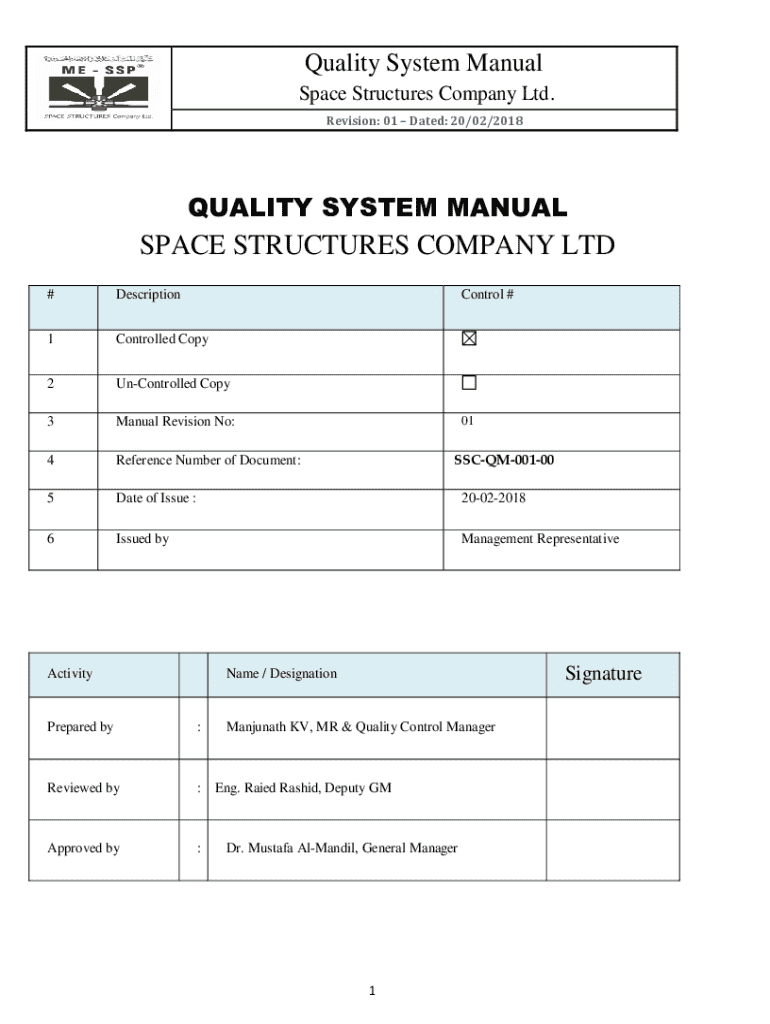
A quality system manual form serves as a crucial document in the framework of a Quality Management System (QMS). It defines the organization’s quality policies, objectives, and processes, thereby establishing standards for performance. The importance of this manual cannot be overstated, as it not only guides employees but also ensures conformance to regulations and industry standards.
The role of a quality system manual form extends beyond internal use; it also facilitates regulatory compliance, illustrating to stakeholders—including regulators, customers, and suppliers—that the organization is committed to maintaining high standards. Key components typically include the company's quality policy, objectives, management responsibilities, and procedures that need to be adhered to.
General information on quality system manuals
Quality Management Systems (QMS) have evolved significantly over the years. Initially, the focus was primarily on product quality, but modern QMS encompass comprehensive strategies that include process integration, customer satisfaction, and continual improvement. Commonly adopted standards like ISO 9001 provide organizations with a framework for establishing an effective QMS.
The purpose of establishing a quality system manual is multifaceted. Firstly, it serves as an official documentation of processes and procedures, acting as a vital reference for training and guiding personnel across the organization. Secondly, it aligns the organization’s quality objectives with its operational goals, ensuring that every employee understands their role in achieving quality.
Structure of a quality system manual
A well-structured quality system manual is essential for clarity and effectiveness. Typical sections include an introduction that outlines the manual's purpose, followed by a detailed scope of the QMS which defines its applicability and boundaries. Management roles and responsibilities should be clearly stated, ensuring accountability and ownership at all levels.
Additional sections may cover the processes involved in the QMS, documentation requirements, and descriptions of how quality objectives are set and measured. This structure not only provides a clear guide for users but also aids in maintaining compliance and meeting regulatory requirements.
Role of the manual in the QMS
The quality system manual plays an integral role in supporting the organization's quality objectives. By documenting processes and timelines, it allows for systematic tracking of performance metrics and facilitates the identification of areas for improvement. Additionally, it integrates seamlessly with other quality documents such as procedures, work instructions, and quality records.
This integration not only ensures that everyone follows a consistent approach in their work but also helps in aligning various quality programs and plans. For example, linking the manual to quality records helps in maintaining traceability of actions taken and decisions made.
Key components of a quality system manual form
A quality policy is paramount within the quality system manual. This defines the organization's commitment to quality and sets the tone for all quality-related activities. Formulating an effective quality policy involves identifying the organization's core values and ensuring that they are reflected in its commitment to quality.
Quality objectives also form a crucial part of the manual. These objectives should be measurable and aligned with the organization’s broader goals. Management commitment is another fundamental element. Leadership must demonstrate their commitment to quality management through active engagement and support—facilitating a culture where quality is prioritized.
Addressing risks and opportunities
Adopting a risk-based approach in quality management allows organizations to proactively identify potential risks that could impact quality. By documenting these risks and outlining actions to mitigate them, organizations can enhance their resilience. For example, risk assessments can be systematically conducted, allowing teams to understand vulnerabilities and devise strategies to address them.
Additionally, capturing opportunities for improvement is essential. The quality system manual should include provisions for recognizing and assessing potential enhancements, empowering teams to make evidence-based decisions that foster continual growth.
Process approach in quality management
The process approach is a foundational concept in quality management, focusing on understanding and managing interrelated processes. This approach encourages organizations to map out key processes, determining inputs, outputs, responsibilities, and interactions between various elements. By embracing a process-focused structure, organizations are well-positioned to optimize their operations.
Continuous improvement remains a crucial aspect of this approach. Organizations should regularly evaluate their processes based on performance metrics and feedback. Adjustments should be made accordingly—a commitment to excellence that not only enhances customer satisfaction but also improves overall efficiency.
Documenting your quality system manual form
Effective documentation is central to a well-functioning quality system manual. Organizations should adopt guidelines that prioritize clarity, consistency, and accuracy throughout the documentation process. Standardization not only enhances comprehension among users but also facilitates easier updates and maintenance.
Common pitfalls include over-complicating language, neglecting to update procedures, and failing to ensure accessibility for both current and new employees. Checklists can be beneficial to ensure all sections are completed correctly and conform to the organization's quality policies.
Editable and collaborative features of quality system manual forms
pdfFiller offers a variety of editing tools that streamline the creation and modification of quality system manuals. Users can easily create, edit, and customize their quality system manual forms to meet their organization's unique requirements. With eSign capabilities included, acquiring approvals from stakeholders becomes a straightforward process.
Collaboration features are particularly beneficial for teams working together on documentation projects. Tools for feedback and commentary within the platform promote input from multiple team members, enhancing the quality of the final document and ensuring that everyone’s expertise is utilized.
Managing your quality system manual
Regular reviews and updates of the quality system manual are critical for maintaining its relevance and compliance. Organizations should establish a schedule for reviewing the manual, assessing its contents against changing regulations and quality standards. A checklist can facilitate this process, outlining key areas to evaluate during each review session.
Archiving older versions of the manual and implementing version control practices prevent confusion and enable teams to refer back to previous documents, ensuring that historical records are maintained without cluttering the current working version.
Tips for effective use of a quality system manual form
Training team members on the quality system manual is fundamental to its success. Organizations should ensure that new hires undergo a thorough introduction to the manual, emphasizing not just document contents but the reasoning behind policies and procedures. Encouraging adherence to documented processes can foster a culture of quality within the organization.
Feedback mechanisms are invaluable for ongoing improvement. Teams should feel empowered to provide input on the manual’s contents, identifying any gaps or suggesting enhancements. By adopting an iterative approach, the quality system manual evolves alongside the organization’s practices.
Interactive tools and resources for quality management
Utilizing platforms like pdfFiller enables organizations to enhance their document management capabilities significantly. Along with quality system manuals, users have access to numerous templates and forms tailored for various quality management needs. These resources can save time and improve consistency across documents.
Case studies of effective quality system implementation showcase how organizations have successfully adapted their manuals using digital tools. These examples demonstrate the potential for increased efficiency, improved compliance, and enhanced team collaboration, ultimately leading to better quality outcomes.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send quality system manual to be eSigned by others?
How do I make changes in quality system manual?
How do I edit quality system manual straight from my smartphone?
What is quality system manual?
Who is required to file quality system manual?
How to fill out quality system manual?
What is the purpose of quality system manual?
What information must be reported on quality system manual?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.