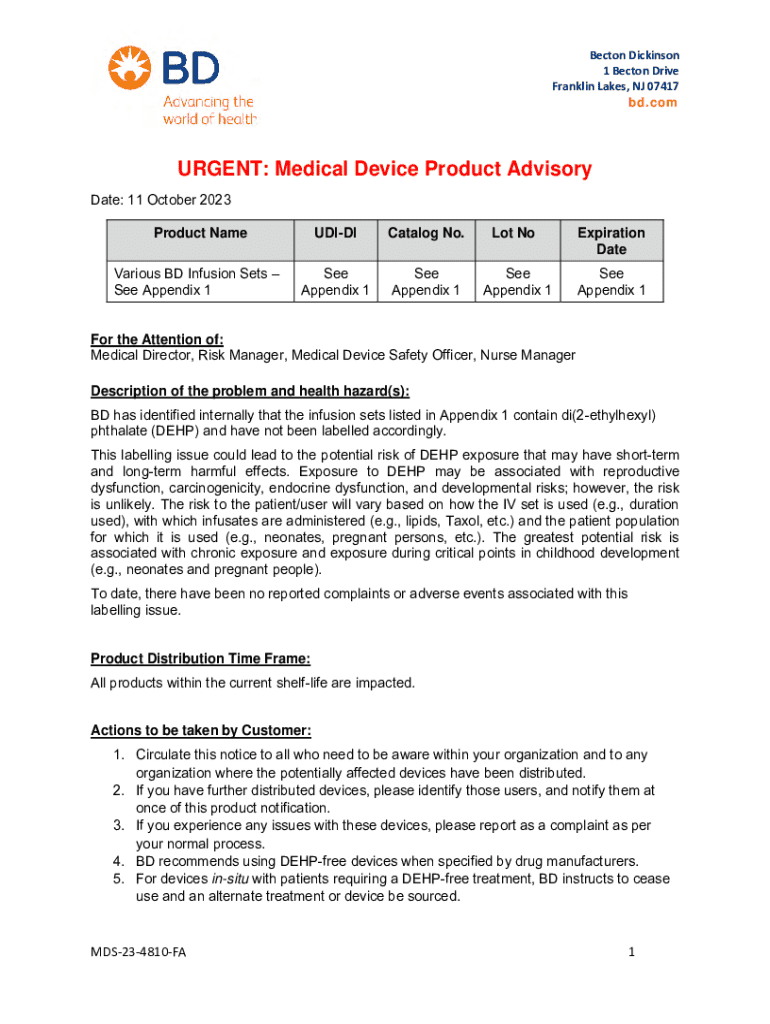
Get the free Medical Device Product Advisory
Get, Create, Make and Sign medical device product advisory



How to edit medical device product advisory online
Uncompromising security for your PDF editing and eSignature needs
How to fill out medical device product advisory

How to fill out medical device product advisory
Who needs medical device product advisory?
Understanding the Medical Device Product Advisory Form: A Comprehensive Guide
Understanding the medical device product advisory form
The medical device product advisory form is a crucial tool in the healthcare industry, serving to communicate essential information regarding specific medical devices. Its primary purpose is to ensure user safety and uphold compliance with regulatory standards. When a potential issue is identified with a device, either related to safety, quality, or compliance, this form acts as the formal notification mechanism, alerting stakeholders about the nature of the problem and the necessary actions to be taken.
Key components of the medical device product advisory form typically include the device identification details, description of the issue, affected user groups, and instructions for managing the advisory. Understanding these components ensures that all relevant information is provided, facilitating prompt and effective communication.
Types of medical device advisories
Medical device advisories can be categorized based on their nature and urgency. The three primary types of advisories include safety advisories, compliance advisories, and quality advisories. Each type serves a unique purpose in the realm of medical device management.
Each advisory type carries specific implications for users, emphasizing the importance of timely and accurate notifications. For instance, a safety advisory may necessitate immediate action from healthcare providers, while a compliance advisory could affect the manufacturer's operational practices.
Legal and regulatory framework
Navigating the legal and regulatory landscape surrounding medical devices is complex. The FDA has established guidelines that define the responsibilities of manufacturers when it comes to alerting users about device issues. Familiarity with these regulations is pivotal for compliance and sustainability in the medical device market.
Non-compliance can lead to severe consequences, including fines, mandatory recalls, and loss of market reputation. Therefore, understanding and adhering to these regulations is essential for manufacturers.
Steps to complete the medical device product advisory form
Filling out the medical device product advisory form involves several critical steps. Proper preparation ensures that all necessary information is collected before the form is completed.
While filling out the form, include precise personal and company information, clear device identification and description, the nature of the advisory, and a detailed distribution plan for the notification. Once completed, thorough review and proper submission channels need to be followed to ensure that the advisory is communicated effectively.
Managing medical device advisories
After submitting the medical device product advisory form, it’s crucial to actively manage the advisory process. This includes monitoring responses from regulatory bodies and maintaining communication with all affected parties, ensuring that they are informed of the steps being taken.
Tracking the efficacy of the advisory can be done through data collection methods and feedback mechanisms to evaluate the response effectiveness and inform future advisories.
The role of digital tools in form management
Digital tools play a pivotal role in managing medical device product advisory forms. Utilizing a platform like pdfFiller can streamline the process from editing to submission.
Moreover, accessing forms from anywhere through a cloud-based solution ensures that all stakeholders can contribute to the advisory process regardless of their location, thereby improving efficiency.
Common challenges and solutions
Completing and managing the medical device product advisory form is not without its challenges. Identifying these common pitfalls can help streamline the process and promote more efficient advisory management.
Best practices to overcome these challenges include utilizing comprehensive checklists to ensure all required components are included and engaging with industry experts or consultants to provide guidance and review during the advisory process.
Case studies and examples
Real-world applications of the medical device product advisory form highlight its significance in the industry. Success stories illustrate how timely advisories have positively impacted patient safety and enhanced compliance.
These case studies emphasize the need for diligence in the advisory process, as the repercussions of poorly managed advisories can lead to escalating risks and liabilities.
Future trends in medical device advisories
As technology and regulatory landscapes evolve, so do the practices surrounding medical device advisories. Anticipated changes in guidelines may affect how advisories are issued and the technology used to manage them.
To prepare for these trends, organizations need to implement strategies that emphasize continuous learning, staying updated on regulatory changes, and adapting their advisory processes accordingly to maintain compliance and safety.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit medical device product advisory from Google Drive?
How do I make changes in medical device product advisory?
Can I create an electronic signature for the medical device product advisory in Chrome?
What is medical device product advisory?
Who is required to file medical device product advisory?
How to fill out medical device product advisory?
What is the purpose of medical device product advisory?
What information must be reported on medical device product advisory?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















