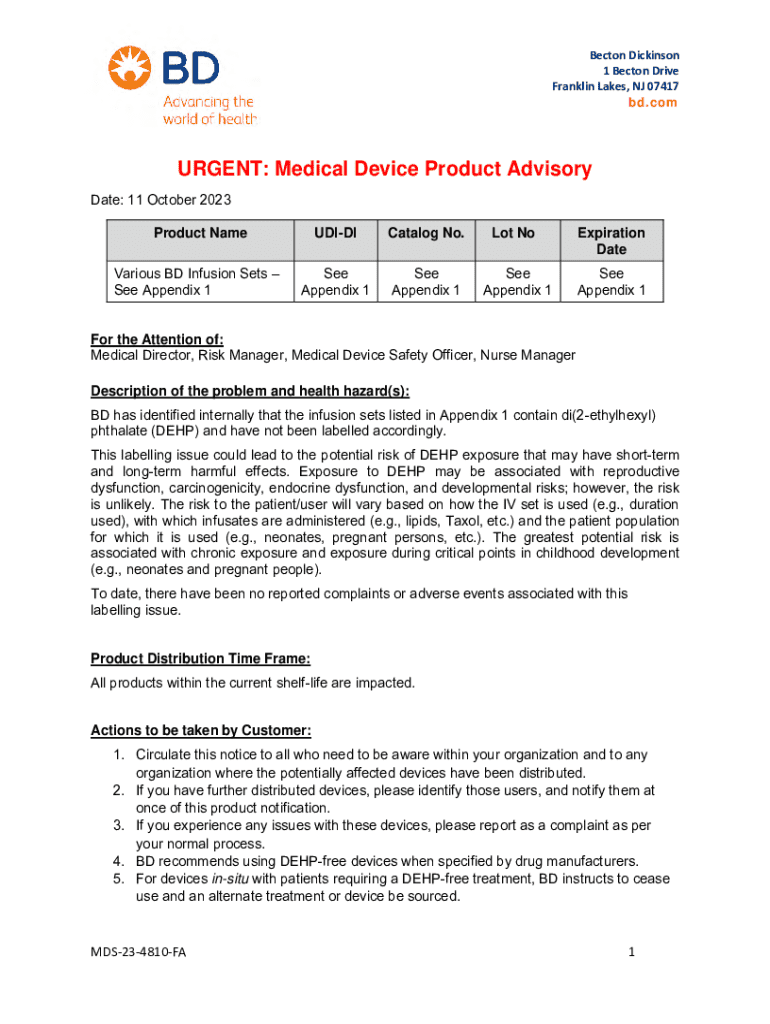
Get the free Medical Device Product Advisory
Get, Create, Make and Sign medical device product advisory



How to edit medical device product advisory online
Uncompromising security for your PDF editing and eSignature needs
How to fill out medical device product advisory

How to fill out medical device product advisory
Who needs medical device product advisory?
Comprehensive Guide to Medical Device Product Advisory Forms
Understanding medical device product advisory forms
A medical device product advisory form is a crucial document in the medical device industry, serving as a formal means of communicating important safety information about medical devices to users, healthcare professionals, and regulatory bodies. Its primary purpose is to inform stakeholders about potential risks, defects, or necessary corrective actions related to specific devices. This form plays a pivotal role in ensuring patient safety and product reliability, balancing the delicate relationship between public health and the manufacturer’s responsibility to uphold industry standards.
In addition to safeguarding public health, medical device product advisory forms have significant legal and compliance implications. They provide documented evidence that manufacturers have adhered to essential reporting requirements established by regulatory bodies such as the FDA. These implications can protect manufacturers against potential lawsuits or liabilities that may arise if a failure to inform users could lead to injury or malfunction.
Key components of a medical device product advisory form
The medical device product advisory form comprises several key components that effectively communicate critical information. Understanding these components is essential for drafting a comprehensive advisory form that meets industry standards and regulatory requirements.
Each component is critical in effectively communicating risks and necessary actions to stakeholders, ensuring they fully comprehend the gravity of the advisory notice.
Step-by-step guide to filling out the medical device product advisory form
Filling out a medical device product advisory form can seem daunting. However, breaking it down into manageable steps can make the process more straightforward and efficient. Here’s a detailed step-by-step guide to navigate the form completion.
Strategies for editing and managing medical device product advisory forms
Effective management of medical device product advisory forms is crucial in maintaining compliance and facilitating quick updates when necessary. Utilizing online tools such as pdfFiller significantly enhances the editing process. These tools allow for effortless edits, annotation, and collaboration across teams, ensuring that everyone involved in the advisory process can contribute their insights.
Best practices for document version control include maintaining a clear naming convention, saving frequently, and ensuring that only authorized individuals edit critical documents. Consistent version tracking will help in managing changes more efficiently.
Collaboration and communication: Engaging stakeholders effectively
Engaging multiple stakeholders during the advisory process is essential. Cross-departmental collaboration facilitates sharing perspectives and expertise, which can lead to more comprehensive and effective advisories. Teams involved typically include Quality Assurance, Regulatory Affairs, and Product Management.
Utilizing tools like pdfFiller can enhance team collaboration with features such as real-time editing and commenting. This interactive environment allows for quicker feedback and streamlined decision-making.
eSigning the medical device product advisory form
Electronic signatures (eSigning) have revolutionized the way legal documents, including medical device product advisory forms, are executed. They provide a legally valid method of obtaining signatures while enhancing efficiency. The use of eSignatures not only speeds up the approval process but also simplifies record-keeping for manufacturers.
By following these steps, the eSigning process becomes secure and efficient, ensuring compliance and maintaining the integrity of the advisory notice.
Managing updates and revisions to advisory forms
Medical device product advisory forms should be viewed as living documents that may require updates as new information becomes available or situations change. Understanding when to revise the document is key. Common scenarios include the identification of new risks, changes in regulatory guidelines, or updates based on user feedback.
Using pdfFiller to streamline revision techniques allows users to efficiently track document history and revisions. This tracking function aids in creating versions of the document that remain accessible for future reference.
Compliance and regulatory considerations
Ensuring compliance with federal and international regulations is paramount when issuing medical device product advisories. The FDA outlines specific requirements that manufacturers must adhere to when submitting advisory notices. Additionally, standards like ISO 13485 demand rigorous documentation and quality management practices.
Manufacturers without proper adherence to these regulations face serious ramifications that could impact not only their market position but also patient health and safety.
Case studies: Effective use of medical device product advisory forms
Examining real-world examples of medical device product advisories highlights their importance in effectively communicating risks and best practices. One notable example is the advisory issued for a popular brand of infusion pumps, where timely communication regarding a software glitch prevented potentially hazardous dosing errors.
These cases exemplify the effectiveness of thorough advisories. Ensuring proper communication can mitigate risks and reinforce trust between manufacturers and users.
Maximizing the utility of medical device product advisory forms
Medical device product advisory forms do more than simply meet compliance standards—they build safety awareness and trust among users. By leveraging tools like pdfFiller’s cloud-based platform, organizations can enhance their document management processes and streamline advisory communications.
Utilizing a comprehensive approach to form management, powered by tools like pdfFiller, ultimately yields significant benefits in both compliance and consumer safety.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in medical device product advisory?
How do I fill out the medical device product advisory form on my smartphone?
How do I complete medical device product advisory on an iOS device?
What is medical device product advisory?
Who is required to file medical device product advisory?
How to fill out medical device product advisory?
What is the purpose of medical device product advisory?
What information must be reported on medical device product advisory?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















