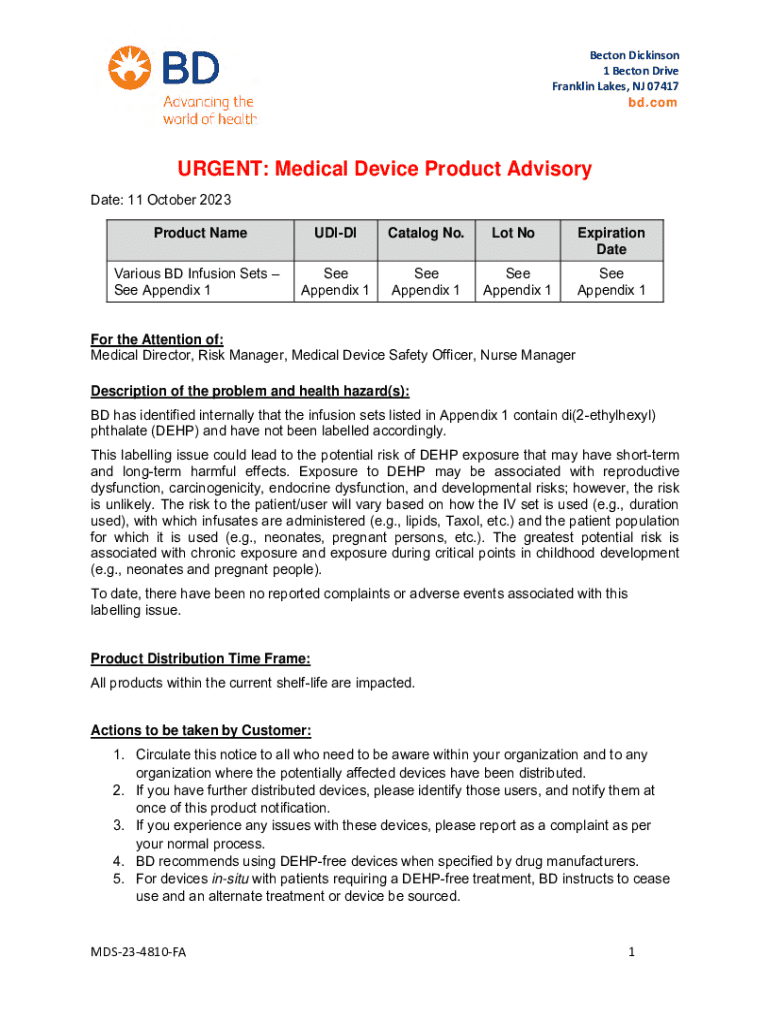
Get the free Medical Device Product Advisory
Get, Create, Make and Sign medical device product advisory



Editing medical device product advisory online
Uncompromising security for your PDF editing and eSignature needs
How to fill out medical device product advisory

How to fill out medical device product advisory
Who needs medical device product advisory?
Your Essential Guide to the Medical Device Product Advisory Form
Understanding the medical device product advisory form
A medical device product advisory form plays a crucial role in maintaining regulatory compliance and ensuring patient safety. This form documents essential information about specific issues regarding medical devices, enabling manufacturers and healthcare providers to communicate effectively about risks and necessary actions. Without such forms, the process of identifying and managing potential device-related problems would be disorganized, leading to complications in patient care.
The importance of advisory forms cannot be understated; they serve as a proactive approach to informing all stakeholders, including healthcare providers, regulatory bodies, and patients, of product issues, thereby enhancing safety protocols and complying with regulations. Proper handling of these forms ensures efficient communication, allowing remedies and involving necessary stakeholders in addressing the identified issues.
Types of medical device advisory notices
Medical device advisory notices serve to alert stakeholders about various issues concerning device safety or performance. While advisory notices are critical for maintaining patient safety, they differ significantly from product recalls. A recall typically involves the retrieval of a product already distributed to users due to safety concerns, while advisory notices may merely inform about potential concerns without the need for product return.
Common types of advisories include safety alerts, which warn about serious risks associated with device use, performance alerts that highlight diminished effectiveness or functionality of a device, and advisory notifications concerning modifications made to improve a product's safety or efficacy. Understanding these distinctions helps stakeholders effectively respond to each type of notice.
The regulatory framework governing advisory forms
Adhering to regulatory frameworks such as ISO 13485 and FDA regulations is integral to the medical device industry. ISO 13485 outlines requirements for establishing a quality management system focusing on consistent compliance and safety. The FDA, representing a significant regulatory body in the United States, provides directives outlining necessary processes for managing product advisories, with clear expectations concerning reporting and compliance.
Global regulatory considerations also impact advisory practices and vary by region. Hence, manufacturers must remain attentive to all relevant guidelines applicable in the markets they serve. Companies that align their advisory processes with these regulations not only protect patients but also safeguard their business interests by minimizing potential legal repercussions.
Step-by-step guide to filling out the medical device product advisory form
Before filling out the medical device product advisory form, it’s essential to gather all necessary documentation, including risk assessments and product attributes. Each detail can significantly impact how the advisory is received and acted upon. Identifying the specific issue and potential risks associated with the device aids in accurately completing the form.
Here’s how to effectively fill out the form: Start with product identification, including the Unique Device Identifier (UDI), which is critical for tracking the device across supply chains. Next, clearly describe the issue at hand, articulating why the advisory is necessary. Assessing the risk involved allows you to provide an informed viewpoint on the matter. Finally, outline recommended actions for stakeholders to follow and include accurate contact information for responses.
Interactive tools for form management
Using a cloud-based platform like pdfFiller can significantly enhance the efficiency of document management regarding advisory forms. Its features allow for collaborative editing, which can minimize errors and ensure all relevant insights are captured in real-time. By leveraging such a platform, teams can edit, eSign, and store forms securely, transforming cumbersome paperwork into manageable digital formats.
Additionally, pdfFiller provides options for version control and tracking changes, which facilitates seamless collaboration among teams. With multiple users able to access the document simultaneously, you can ensure that everyone stays updated on changes and is poised to act on the advisory swiftly.
Common challenges and how to overcome them
Navigating the complex regulatory requirements for medical device advisories can be daunting. Developers and manufacturers must ensure they meet all regulatory demands to avoid potential fines or legal ramifications. One way to mitigate these challenges is by conducting comprehensive training for all team members involved in filling out the advisory forms on current regulations and company protocols.
Moreover, clarity and precision are paramount when communicating through advisories. Keeping language straightforward and avoiding medical jargon can make the advisory more comprehensible for all stakeholders. Addressing stakeholder concerns upfront by developing a communication strategy also allows for efficient response to any questions or issues that may arise.
Best practices in managing medical device recalls and advisories
Managing medical device recalls and advisories adeptly involves a blend of proactive and reactive approaches. A proactive stance includes regular audits and risk assessments, allowing companies to address issues before they escalate. Conversely, being reactive means adapting quickly to unforeseen problems as they arise, utilizing forms that facilitate immediate communication.
Additionally, thorough documentation during the advisory process is critical for audits and regulatory purposes. Keeping comprehensive records can demonstrate compliance and facilitate response during inspections. Finally, effectively communicating updates about advisories to all affected parties is essential for maintaining trust and ensuring the continued safety of patients.
Importance of continual training and education
Continual training and education are vital in ensuring that all personnel involved with medical device advisories are well-informed about compliance standards. Regular training sessions can keep teams updated on changing regulations and best practices related to advisory forms, enhancing the overall competency of your workforce. Investing in such programs not only builds trust among stakeholders but also safeguards patient safety.
Staying updated with regulatory changes should be a priority for all organizations involved in medical device management. Subscribing to relevant journals, attending industry conferences, and utilizing online courses such as those offered by regulatory agencies can help personnel stay informed and compliant.
Tools for continuous improvement
Continuous improvement in managing medical device product advisories involves listening to feedback from stakeholders. Following up after advisories are issued can provide insights that inform future processes and best practices. Systematic feedback mechanisms can help identify areas that may need refinement, whether in communication or processes.
Analytics play a pivotal role in evaluating advisory effectiveness and areas for enhancement. Leveraging data to track results from advisories can shine a light on response times, understanding of issues, and stakeholder satisfaction. Using these insights can empower organizations to evolve their advisory processes consistently.
Addendum: Using pdfFiller for optimal document management
pdfFiller stands out as an optimal tool for managing medical device product advisory forms. Its unique features cater specifically to the needs of professionals in the medical device field. The platform’s ability to streamline the document management process significantly reduces errors and enhances efficiency in filling, signing, and sharing forms.
Testimonials from users of pdfFiller underscore its effectiveness in minimizing downtime and improving interdepartmental communication. Case studies have shown that teams can respond faster to advisory requirements and manage documents much more efficiently when using a collaborative tool like pdfFiller.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit medical device product advisory online?
How do I edit medical device product advisory straight from my smartphone?
How do I edit medical device product advisory on an Android device?
What is medical device product advisory?
Who is required to file medical device product advisory?
How to fill out medical device product advisory?
What is the purpose of medical device product advisory?
What information must be reported on medical device product advisory?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















