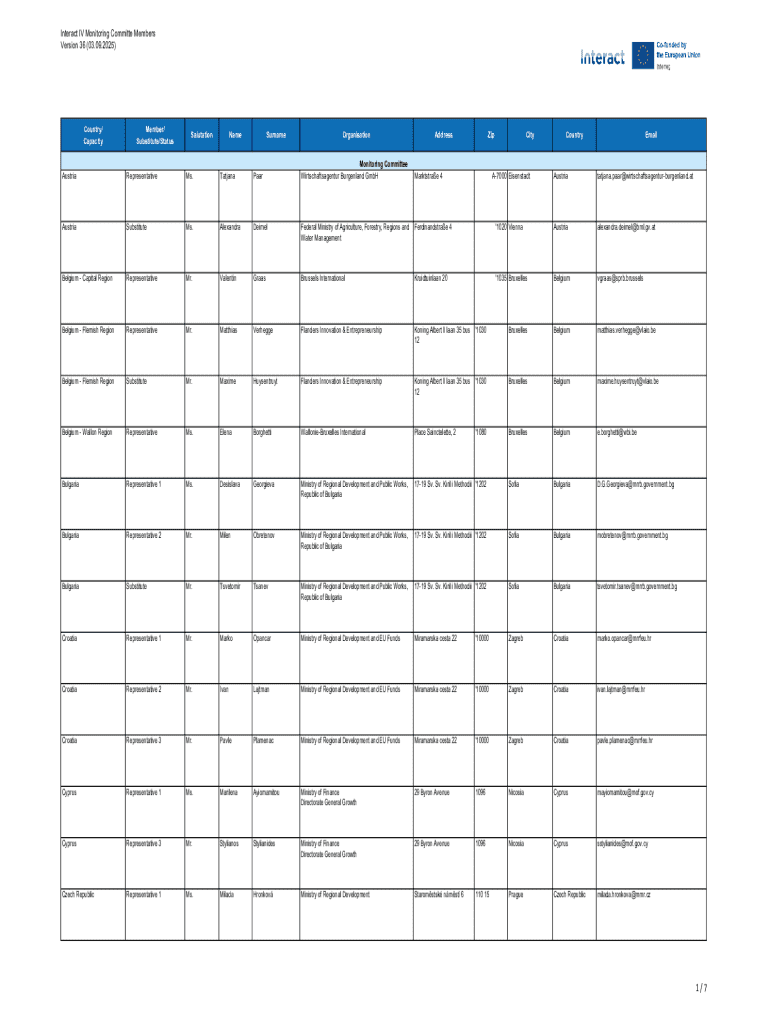
Get the free Interact Iv Monitoring Committe Members
Get, Create, Make and Sign interact iv monitoring committe



How to edit interact iv monitoring committe online
Uncompromising security for your PDF editing and eSignature needs
How to fill out interact iv monitoring committe

How to fill out interact iv monitoring committe
Who needs interact iv monitoring committe?
A comprehensive guide to the Interact Monitoring Committee Form
Understanding the Interact Monitoring Committee Form
The Interact IV Monitoring Committee Form is an essential document used in various oversight and research contexts, specifically focusing on patient interactions and outcomes. Its primary purpose is to ensure that all monitoring activities regarding clinical trials or health-related assessments are systematically recorded and reviewed. The importance of this form cannot be overstated as it acts as a cornerstone for quality assurance in clinical protocols.
Key stakeholders involved with this form often include clinical researchers, project managers, and institutional review boards. These professionals rely on the data collected via the Interact IV Monitoring Committee Form to assess compliance with regulatory standards, evaluate the effectiveness of interventions, and enhance overall patient safety.
Scope and applicability of the form
This form is tailored primarily for researchers and organizations conducting clinical trials that require oversight from a monitoring committee. Individuals and teams responsible for patient data collection, management, and reporting are the principal users of the Interact IV Monitoring Committee Form. Situations necessitating submission can range from periodic monitoring assessments to immediate reporting of significant findings or adverse events.
Detailed breakdown of the Interact Monitoring Committee Form
Completing the Interact IV Monitoring Committee Form involves carefully considering each section and the information required. This ensures that every necessary aspect of monitoring activities is captured effectively to facilitate thorough review and compliance.
Section-by-section analysis
Starting off, the personal information section requires complete and accurate data, including names, titles, and contact information. Common pitfalls include incomplete entries or typographical errors, which could lead to delays in the review process.
The committee member details section necessitates an accurate listing of all members involved in the monitoring process. It’s crucial that this information is carefully verified to avoid any miscommunication or withdrawal of necessary approvals.
Next, the monitoring activities overview is essential for detailing the type of monitoring performed, including any findings or interventions considered. Including clear examples of monitoring activities can bolster the quality of the submission and provide context for evaluations.
Finally, reporting metrics are instrumental in assessing the efficiency and effectiveness of interventions. Choosing the right metrics is vital; they should be aligned with the core objectives of the monitoring committee. Tips for effective reporting include utilizing clear visuals and summaries that highlight major findings.
Step-by-step guide to completing the form
To streamline the completion process of the Interact IV Monitoring Committee Form, you first need to gather all necessary documentation. Compiling required information beforehand is essential for efficiency and accuracy.
Preparing your documentation
Gathering all relevant data, such as patient records, intervention summaries, and team member roles, is crucial. Having this documentation organized can save you time during submission. Utilizing tools like PDFfiller aids in document management, allowing for easy organization and retrieval.
Filling out the form
When filling out the form, adhere to best practices by following each section's specific requirements. Carefully read the instructions to ensure every necessary element is included. Avoid common mistakes like procrastination or rushing, which can lead to oversights or errors in your submission.
Reviewing and editing your submission
It’s crucial to double-check all entered information before submitting the form. Collaboration features in PDFfiller enable team members to review and edit submissions, ensuring accuracy and compliance with the required standards.
Utilizing interactive tools for enhanced workflow
Embracing cloud-based solutions provided by PDFfiller revolutionizes how you manage and submit the Interact IV Monitoring Committee Form. A centralized document management system simplifies filing processes and ensures that all documents are easily accessible, promoting a smoother workflow.
eSigning and validating your submission
Utilizing electronic signatures offers a contemporary approach to ensuring submission validity. By using trusted eSigning solutions, you can ensure compliance with legal and organizational regulations. Understanding the functionality of various digital signing processes can significantly expedite your document workflows.
Managing and storing your completed form
Once the Interact IV Monitoring Committee Form is completed and submitted, proper document management becomes imperative. Adopting best practices for digital storage involves organizing files in a logical manner and ensuring that sensitive information is safeguarded with appropriate security measures.
Digital storage options
Consideration should also be given to future access and edits of the form. Knowing how to retrieve and reuse previous versions saves time in future submissions. Efficient document organization systems help track changes and facilitate updates, enhancing overall ease of use.
FAQs about the Interact Monitoring Committee Form
Familiarizing yourself with common questions regarding the Interact IV Monitoring Committee Form can significantly reduce confusion during the completion process. Establishing a resource for answers helps streamline team discussions and reduces repetitive inquiries.
Common questions and answers
Examples of FAQs can include inquiries about specific reporting metrics appropriate for various research contexts or clarifications on submission deadlines and compliance requirements. Setting up a detailed FAQ section can address typical doubts and improve the experience of users working with the form.
Troubleshooting tips for submission issues
When facing submission challenges, users often find navigating system errors or document incompatibilities frustrating. Offering straightforward troubleshooting steps, such as checking file compatibility or ensuring connectivity, can make the submission process smoother.
Feedback and improvements
Gathering feedback from users on the Interact IV Monitoring Committee Form is instrumental in enhancing its usability and effectiveness. Establishing avenues for feedback encourages continuous improvement, which is vital for adapting to changes in the regulatory landscape or operational needs.
How to provide feedback on the form
Encouraging users to share their experiences can yield insights that inform future revisions. Providing straightforward mechanisms for feedback collection, such as surveys or comment sections, fosters a culture of collaboration and refinement within the document management process.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send interact iv monitoring committe for eSignature?
How do I execute interact iv monitoring committe online?
How do I edit interact iv monitoring committe straight from my smartphone?
What is interact iv monitoring committee?
Who is required to file interact iv monitoring committee?
How to fill out interact iv monitoring committee?
What is the purpose of interact iv monitoring committee?
What information must be reported on interact iv monitoring committee?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















