Get the free Regulation (eu) 2024/… - data consilium europa
Get, Create, Make and Sign regulation eu 2024



Editing regulation eu 2024 online
Uncompromising security for your PDF editing and eSignature needs
How to fill out regulation eu 2024

How to fill out regulation eu 2024
Who needs regulation eu 2024?
Regulation EU 2024 Form: How-to Guide
Understanding Regulation EU 2024: Key objectives and implications
Regulation EU 2024 is a significant development in the regulatory landscape, primarily impacting sectors such as medical devices and in vitro diagnostics. The regulation serves to enhance patient safety and streamline compliance processes, making it crucial for manufacturers to understand its implications. With a stronger focus on post-market surveillance and transparency, this regulation aims to address some of the critical issues previously overlooked in earlier frameworks. Compliance with these new mandates is fundamentally important, as it pairs safety priorities with efficiency for health systems across Europe.
The timeline for implementation is essential for all stakeholders. Key dates include the regulation's official publication, expected by mid-2024, with full compliance required by 2026. Transitional provisions will allow manufacturers some leeway in adhering to new requirements, but timely action is essential to ensure no lingering issues arise as deadlines approach.
Navigating the new regulatory framework
The new compliance landscape under Regulation EU 2024 introduces comprehensive changes that necessitate attention. This helps organizations preparing for future evaluations stay in tune with evolving standards. Compared to previous regulations, such as MDR 2017/745 and IVDR 2017/746, this regulation emphasizes more stringent clinical evaluation requirements and clearer communication obligations. Manufacturers need to adapt their strategies accordingly, ensuring that they stay compliant.
How to complete and submit the Regulation EU 2024 forms
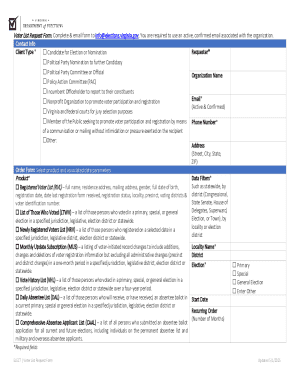
Every manufacturer affected by Regulation EU 2024 needs to familiarize themselves with the new forms required for compliance. Key documentation includes the Manufacturer’s Declaration and Clinical Evaluation Report. Each of these forms serves a vital role in ensuring that products meet safety and efficacy standards before entering the market.
Completing these forms requires careful attention to detail. For example, the Manufacturer’s Declaration must clearly state product information, compliance with relevant standards, and the manufacturer's quality control processes. The Clinical Evaluation Report, on the other hand, should provide comprehensive data on clinical findings, emphasizing patient outcomes and safety.
Tools and resources for document management
To streamline compliance efforts with Regulation EU 2024, using a document management system like pdfFiller can significantly enhance efficiency. It allows users to upload, edit, and securely manage PDF documents from any location. This cloud-based platform empowers teams by facilitating easy collaboration and document sharing, ensuring everyone stays aligned on compliance requirements.
Additionally, pdfFiller’s interactive features can greatly improve the document workflow. Tools such as eSigning and version control help maintain compliance and keep track of changes throughout the lifecycle of a document. This is especially beneficial in the highly regulated health sector, where accuracy and timely updates are paramount.
Compliance and quality assurance measures
To ensure compliance with Regulation EU 2024, manufacturers should adopt best practices tailored to the new requirements. Regular internal audits can help identify potential gaps in adherence to compliance, providing an opportunity for proactive measures. Moreover, implementing a quality management system aligned with the regulation can facilitate the continual assessment of compliance and risk management.
Organizations must also be aware of common pitfalls in the documentation and compliance processes. Misinterpreting regulatory requirements can lead to costly compliance issues, and documentation errors may result in delays or rejections. Therefore, training sessions for teams on regulatory updates and best practices are invaluable.
Case studies and real-life applications
Exploring successful implementations of Regulation EU 2024 can provide insights and inspire best practices among organizations. Companies that have excelled in their compliance efforts often demonstrate proactive strategies, such as the incorporation of advanced compliance management technologies and thorough training programs that educate teams on regulatory updates and requirements.
For instance, a leading medical device manufacturer adopted a robust document management solution from pdfFiller, enabling them to seamlessly create, edit, and store their regulatory documentation. This allowed them to respond quickly to regulatory queries and facilitated smoother audits, significantly reducing compliance risks and enhancing their market reputation.
Regulatory updates and future developments
Staying informed about changes in Regulation EU 2024 is crucial for manufacturers to maintain compliance and adapt to new challenges. Regularly checking for updates from official EU websites or industry-specific forums can provide valuable insights and announcements regarding amendments or clarifications.
Additionally, participating in continuous education and training programs will help teams remain updated on regulatory innovations. This proactive approach ensures that organizations are not only compliant but also well-prepared for future modifications in the regulatory framework.
Frequently asked questions (FAQs) about Regulation EU 2024
As stakeholders navigate the complexities of Regulation EU 2024, a range of common queries arises regarding compliance processes. Many individuals wonder what constitutes sufficient evidence for clinical evaluations or how to interpret specific compliance requirements.
Addressing these questions is essential to demystify the regulation and ensure that all parties involved understand the intricacies. Clarifying misconceptions around deadlines and documentation requirements can significantly ease the overall compliance journey.
Engaging with the regulatory community
Engagement with the regulatory community is vital for individuals and organizations seeking to influence future developments in Regulation EU 2024. By attending conferences and workshops, stakeholders can provide feedback and share experiences that can directly impact the regulation's evolution.
Moreover, participating in online forums and professional groups fosters a shared environment where regulators and industry participants can collaborate. Contributing valuable insights or experiences helps shape the trajectory of future regulatory frameworks.
Conclusion: Empowering your document journey
Efficient document management plays a pivotal role in navigating Regulation EU 2024 requirements. Leveraging advanced platforms like pdfFiller not only simplifies the process of filling out required forms but ensures enhanced collaboration among teams -- where compliance needs align with their priorities.
In conclusion, embracing a comprehensive document management strategy can significantly empower organizations to remain compliant, avoid missteps, and foster a culture of quality assurance in alignment with EU regulations.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in regulation eu 2024?
How do I fill out the regulation eu 2024 form on my smartphone?
How do I complete regulation eu 2024 on an iOS device?
What is regulation eu?
Who is required to file regulation eu?
How to fill out regulation eu?
What is the purpose of regulation eu?
What information must be reported on regulation eu?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.