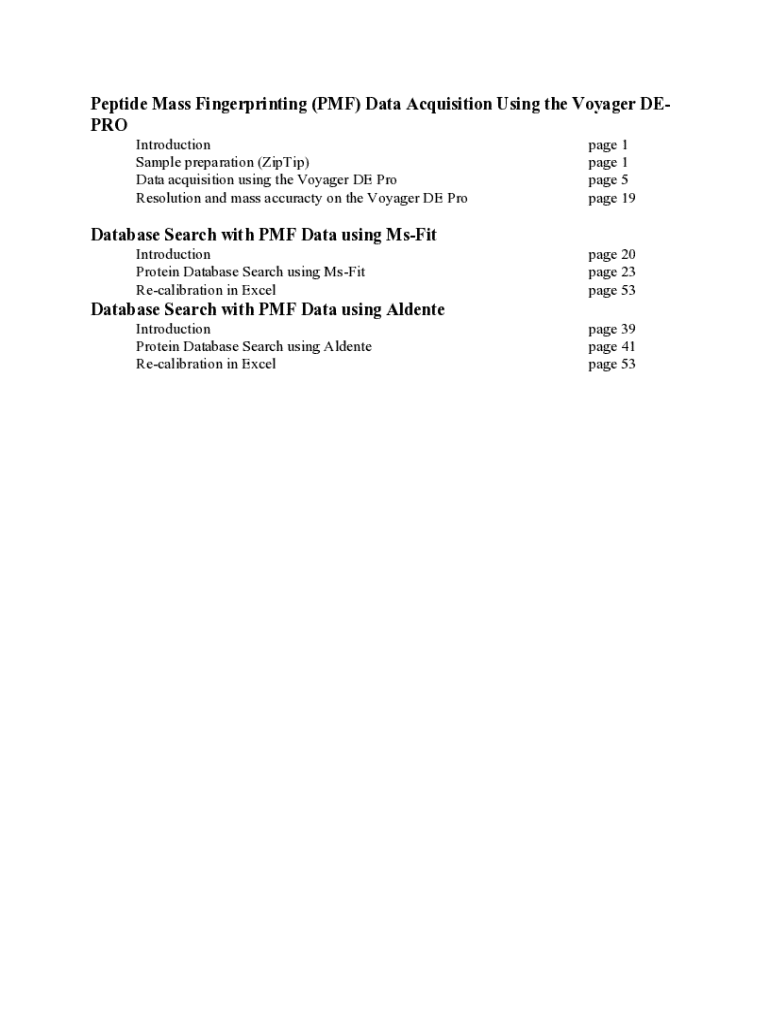
Get the free High-accuracy peptide mass fingerprinting using peak ...
Get, Create, Make and Sign high-accuracy peptide mass fingerprinting

How to edit high-accuracy peptide mass fingerprinting online
Uncompromising security for your PDF editing and eSignature needs
How to fill out high-accuracy peptide mass fingerprinting
How to fill out high-accuracy peptide mass fingerprinting
Who needs high-accuracy peptide mass fingerprinting?
High-accuracy peptide mass fingerprinting form
Overview of high-accuracy peptide mass fingerprinting
High-accuracy peptide mass fingerprinting (PMF) is a crucial analytical technique employed in proteomics to identify proteins based on their mass spectra. This method enables researchers to achieve remarkable precision when characterizing complex protein samples, leading to significant advancements in biological research, clinical diagnostics, and therapeutic developments.
The precision of mass measurements dramatically influences the identification capability of PMF, making high-accuracy measurements an essential aspect of the technique. The ability to pinpoint specific peptide masses accurately ensures better discrimination among similar proteins, hence enhancing the identification reliability across various applications, including biomarker discovery and protein interaction studies.
Key components of the peptide mass fingerprinting process
The PMF process is multifaceted and comprises several critical steps, starting with sample preparation. Sample quality is paramount for ensuring meaningful outcomes. Minimum contaminants and optimal protein concentration lead to better mass spectral data, which are pivotal for accurate analysis. Typically, proteins are isolated from biological samples using a range of sample extraction methods such as precipitation, chromatography, or ultrafiltration, depending on the matrix and intended analysis.
Mass spectrometry (MS) principles are vital for determining the mass-to-charge ratios of ionized peptides. Two common types of mass spectrometry utilized in PMF are Matrix-Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF) and Electrospray Ionization (ESI). Each of these methodologies optimizes the generation of ionized peptides, setting the stage for accurate mass analysis.
Understanding the peak list
A peak list is the cornerstone of the PMF analysis, serving as a compiled representation of the detected peptide masses acquired during mass spectrometry. Accurately identifying peaks within the mass spectrum is critical, as it directly impacts the quality of data used for protein identification. Each peak in the peak list corresponds to a specific peptide or fragment, providing pivotal information on the molecular composition of the sample.
Generating a peak list involves data acquisition directly from the mass spectrometer's instrument data system. Sophisticated software tools are then employed to analyze this raw data and extract peak information efficiently. Various software options, such as Mascot or ProteinPilot, are commonly used to facilitate peak list generation and subsequent database searches.
Search parameters: fine-tuning results
Configuring search parameters is integral for enhancing PMF results. This includes refining specifications for search algorithms that decode the identified peaks against protein databases. Mass tolerances must be set thoughtfully. A smaller tolerance can yield higher specificity in results, whereas a larger tolerance may capture broader potential matches, albeit with reduced relevance.
Understanding the distinctions between fixed and variable modification parameters also plays a crucial role. Fixed modifications, such as carbamidomethylation of cysteine, remain constant for all peptides. In contrast, variable modifications (like oxidation of methionine) can differ among peptides, necessitating careful consideration during the analysis process.
Choosing a sequence database
The selection of a suitable sequence database is pivotal for accurate protein identification in PMF. Researchers often must choose between public databases like UniProt, which offer robust collections of protein sequences across various organisms, or private databases, which may include proprietary or organism-specific sequences tailored to specific studies.
Criteria for selecting a database include the completeness of the entries, the relevance to the target organism, and the updates in the reference sequences. A larger database may provide more potential hits, but an emphasis on quality over sheer size ensures greater reliability in identification results, especially in complex proteomic samples.
Enzymatic digestion considerations
Enzymatic digestion is a critical step in preparing proteins for PMF analysis, with trypsin being the most widely used enzyme due to its specificity for lysine and arginine residues. Understanding the specificity and activity of the chosen enzyme is imperative, as differing cleavage patterns can lead to variations in the resulting peptide profiles and, consequently, the mass spectra.
However, potential pitfalls exist in enzymatic digestion. Incomplete digestion can result in larger protein fragments being present, complicating peak identification and leading to ambiguous results. Therefore, optimizing digestion conditions, such as enzyme concentration and incubation time, is vital to ensuring efficient and reproducible peptide generation.
Common modifications and their impact
Many modifications occur post-translationally and can significantly affect the analysis of PMF data. Fixed modifications, such as the carboxymethylation of cysteine, influence the peptide mass consistently across samples, whereas variable modifications, such as phosphorylation or methylation, are adaptable and can vary depending on cellular conditions.
Handling modifications correctly during PMF data analysis is essential. Failing to account for the presence of variable modifications may obscure potential identification hits or lead to incorrect interpretations. Thus, utilizing robust software that can accurately account for variability ensures more reliable identification of proteins.
Calculating protein mass accurately
Accurate mass calculation is fundamental in PMF as it directly influences the identification process. Several factors can affect the measurement of protein mass, including the charge state's contribution and the presence of any modifications. Techniques such as calibrating mass spectrometers with standard proteins help achieve reliable mass values.
Ultimately, accurate mass calculations not only maximize identification success but also facilitate the quantitative analysis of proteins across different experimental conditions. When doing so, researchers must maintain careful records of the conditions influencing these calculations to ensure reproducibility in future experiments.
Interpreting results: best practices
Analyzing and interpreting results from a PMF study requires careful consideration. A systematic approach involves validating the identification results against known standards, ensuring confidence in the findings. It is critical to assess the alignment between experimental data and theoretical predictions, as well as checking the variation in results across replicates.
Additionally, leveraging software tools that simplify the analysis process can enhance productivity and accuracy. User-friendly interfaces allow teams to visualize mass spectral data effectively and corroborate findings from multiple experimental runs, ensuring that results are both reliable and reproducible.
Interactive tools and resources for users
pdfFiller serves as an invaluable resource for teams engaged in peptide mass fingerprinting. Its document management features allow researchers to create, edit, sign, and collaborate on essential documentation from a centralized, cloud-based platform. By utilizing pdfFiller, users can streamline their workflows, making the management of PMF reports and templates efficient and effective.
Another notable capability of pdfFiller is its proactive support for users. The platform also facilitates seamless access to various forms that can be tailored for specific research needs, such as sample preparation documentation or analysis summaries. Ensuring researchers have the tools they need to optimize their workflow contributes to higher productivity and better scientific output.
FAQs: common questions around peptide mass fingerprinting
The journey through peptide mass fingerprinting often presents challenges. Common issues include incomplete digestion, ambiguous results, and difficulties in data interpretation. To troubleshoot these concerns, researchers should consider revisiting sample preparation steps and ensuring that all parameters are well-defined, from enzymatic digestion to mass spectrometry settings.
When encountering ambiguous results, leveraging collaborative platforms like pdfFiller helps maintain comprehensive records and share findings conveniently, fostering a team's collective troubleshooting process. This not only enhances understanding but also accelerates the identification and resolution of issues specific to PMF practices.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my high-accuracy peptide mass fingerprinting in Gmail?
How can I get high-accuracy peptide mass fingerprinting?
Can I create an electronic signature for signing my high-accuracy peptide mass fingerprinting in Gmail?
What is high-accuracy peptide mass fingerprinting?
Who is required to file high-accuracy peptide mass fingerprinting?
How to fill out high-accuracy peptide mass fingerprinting?
What is the purpose of high-accuracy peptide mass fingerprinting?
What information must be reported on high-accuracy peptide mass fingerprinting?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.