Get the free Biosafety Cabinet Risk Assessment Form
Get, Create, Make and Sign biosafety cabinet risk assessment



How to edit biosafety cabinet risk assessment online
Uncompromising security for your PDF editing and eSignature needs
How to fill out biosafety cabinet risk assessment

How to fill out biosafety cabinet risk assessment
Who needs biosafety cabinet risk assessment?
Biosafety Cabinet Risk Assessment Form: A Comprehensive Guide
Understanding biosafety cabinets
Biosafety cabinets (BSCs) are vital equipment in laboratory environments, designed to protect lab personnel, the environment, and the samples being handled. These specialized enclosures provide a sterile working environment and prevent contamination from hazardous agents. They are essential in settings such as microbiology labs, clinical laboratories, and research facilities that handle infectious materials and potentially dangerous biological substances.
The primary role of a biosafety cabinet is to contain biohazards while maintaining a sterile field for the procedures being performed. Their importance cannot be overstated, as they serve as a barrier that shields laboratory staff from harmful agents and prevents the unintentional release of these materials into the environment. Depending on the level of protection needed, there are different classes of biosafety cabinets.
The need for a biosafety cabinet risk assessment
Conducting a risk assessment for biosafety cabinets is critical to identifying potential hazards within laboratory settings. Regular risk assessments not only fulfill legal and regulatory obligations but also help in effectively protecting personnel and the surrounding ecosystem. In many jurisdictions, compliance with biosafety standards is mandatory, making these assessments a requisite part of the laboratory's operational protocols.
Assessments should be performed periodically, particularly during significant changes such as the introduction of new hazardous materials or modifications to laboratory procedures. Additionally, any incidents or near-misses related to biosafety practices warrant an immediate reassessment to prevent future occurrences. The overarching goal is to mitigate risks by ensuring that appropriate procedures are in place.
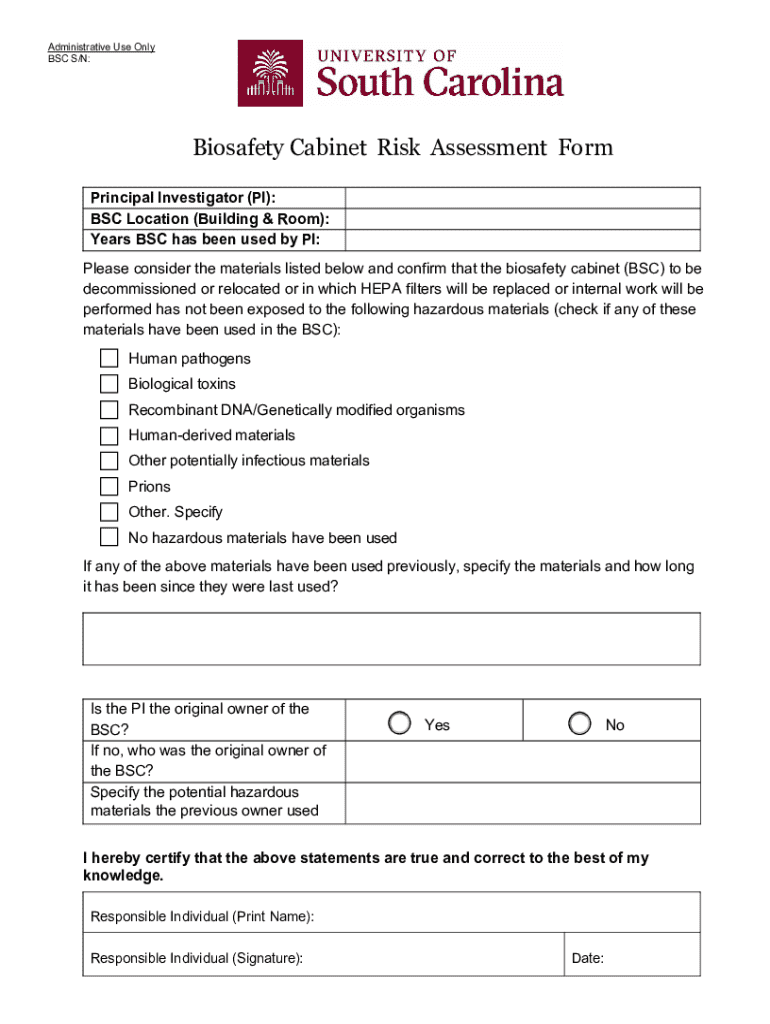
Components of the biosafety cabinet risk assessment form
A biosafety cabinet risk assessment form must be comprehensive, capturing essential details that provide clarity regarding the cabinet's operation and associated risks. The form typically starts with identifying the specific type of biosafety cabinet in use, as this guides subsequent evaluation points. Additionally, recording the cabinet's location and the context in which it will be used is crucial for tailoring safety protocols.
Next, the form should include sections focused on identifying risks. Information about the biological materials being handled should be specified, including their classification and the threat level they pose. Moreover, it's essential to document personnel training and qualifications, as well as any protective measures already in place to ensure safety.
Step-by-step guide to completing the risk assessment form
Completing the biosafety cabinet risk assessment form involves several systematic steps to ensure thorough evaluation and planning.
Interactive tools and resources for risk assessment
Utilizing interactive tools can significantly streamline the risk assessment process for biosafety cabinets. Cloud-based document solutions, like pdfFiller, enable teams to collaborate effectively, allowing for real-time updates and collective input on risk evaluation forms. This enhances the overall quality of assessments and ensures that all pertinent information is captured accurately.
Furthermore, interactive checklists and readily available templates provide valuable starting points for users, ensuring that essential criteria are not overlooked. By leveraging these tools, teams can improve workflow efficiency and comply with safety regulations, ultimately resulting in a more effective risk management strategy.
Best practices for maintaining biosafety cabinets
Maintaining biosafety cabinets is paramount to ensure effective containment and safety in laboratory settings. Regular servicing and certifications by qualified personnel should be scheduled to confirm that cabinets meet operational standards and safety guidelines. This includes testing HEPA filters, verifying airflow patterns, and ensuring that all mechanical systems function properly. Making this a routine practice prevents potential hazards.
In addition to maintenance, adhering to strict cleaning and decontamination protocols post-experimentation is necessary to mitigate cross-contamination risks. Also, ensuring that all personnel members undergo thorough training on biosafety cabinet usage is essential for compliance and safety adherence, equipping them with the knowledge necessary to operate these systems confidently.
Common challenges in risk assessment and solutions
Despite growing awareness of the importance of risk assessments for biosafety cabinets, certain challenges persist. One prevalent issue includes the misidentification of hazards, which can stem from outdated protocols or lack of comprehensive training. It's crucial for laboratories to foster an environment that prioritizes continuous education, ensuring that personnel remain aware of current best practices and emerging risks.
Another challenge is the submission of incomplete or inaccurate forms. A systematic approach to reviewing risk assessments can significantly reduce this occurrence. Implementing peer reviews or collaborative sessions during the assessment process can enhance accuracy and facilitate effective communication among team members, ultimately promoting a culture of safety and diligence in biosafety practices.
Real-world case studies: risk assessments in action
Real-world case studies can provide invaluable insights into effective risk assessment and management strategies. For instance, a prominent university laboratory faced a biosafety breach due to inadequate risk evaluation practices. By revisiting their assessment protocols, they developed a checklist that improved their evaluation accuracy, addressed previously unrecognized hazards, and ultimately strengthened their biosafety measures. This re-evaluation proved beneficial, preventing potential exposure incidents.
Learning from previous incidents underscores the need for continuous improvement in risk assessment. By analyzing the specifics of incidents related to biosafety cabinets, laboratories can create informed strategies that reduce risk and enhance personnel safety. It is imperative that laboratories embrace a proactive culture around risk assessments.
Leveraging PDF solutions for document management
Utilizing pdfFiller for managing biosafety cabinet risk assessment forms offers significant advantages. This platform facilitates easy editing and collaboration, allowing teams to make real-time changes to documents. The integrated eSigning feature streamlines the approval process, enabling responsible individuals to securely sign off on completed forms without unnecessary delays.
Moreover, pdfFiller ensures that all document changes are tracked meticulously. Users can view the history of edits, which is essential when fine-tuning critical safety documents like risk assessments, ensuring that everyone stays informed of the latest iterations. This boosts accountability within teams and underscores the importance of proper document management.
Future trends in biosafety and risk assessment
The field of biosafety is evolving, with emerging technologies and regulatory changes continuously shaping best practices. Innovations in biosafety cabinet technology are making strides in improving containment features, enhancing usability, and integrating digital functionalities for monitoring and data collection. This shift creates a more responsive laboratory environment, capable of adapting to new challenges.
Additionally, predictive risk assessment tools are gaining traction, harnessing data analytics to anticipate potential hazards before they arise. Regulatory bodies are also updating guidelines to reflect these advancements, encouraging laboratories to stay ahead of compliance. Embracing these trends is essential for maintaining effective biosafety practices in research and clinical environments.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in biosafety cabinet risk assessment?
Can I create an electronic signature for signing my biosafety cabinet risk assessment in Gmail?
How do I complete biosafety cabinet risk assessment on an Android device?
What is biosafety cabinet risk assessment?
Who is required to file biosafety cabinet risk assessment?
How to fill out biosafety cabinet risk assessment?
What is the purpose of biosafety cabinet risk assessment?
What information must be reported on biosafety cabinet risk assessment?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.