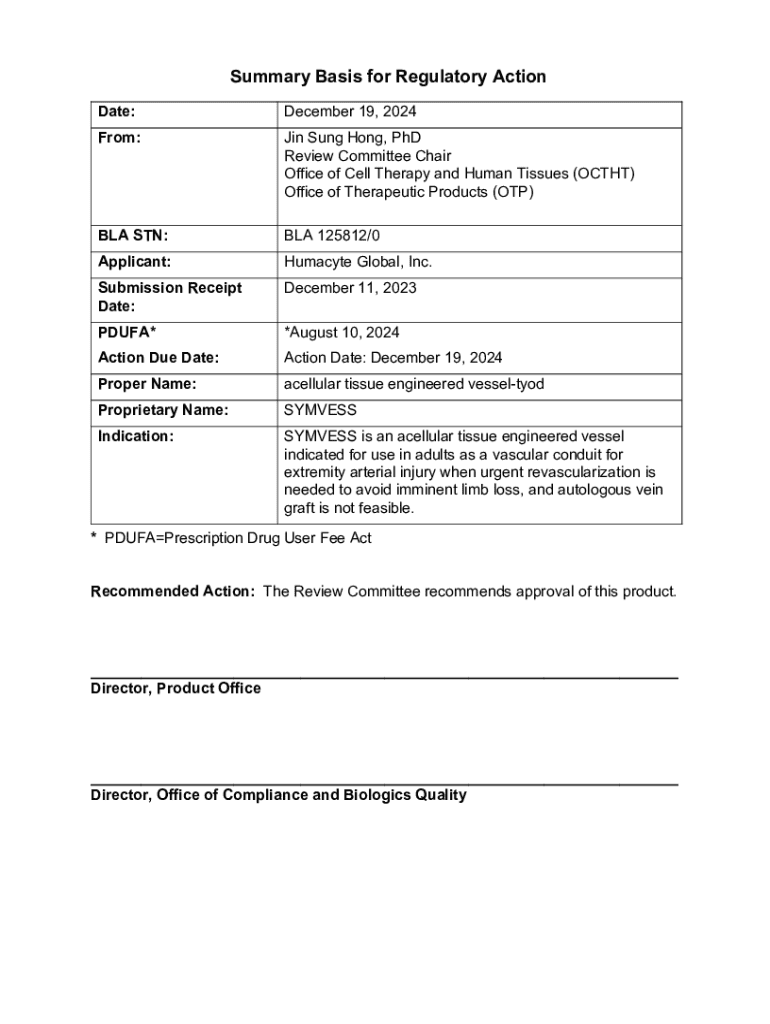
Get the free Regulatory Considerations for Early-Stage Cell and Gene ...
Get, Create, Make and Sign regulatory considerations for early-stage



How to edit regulatory considerations for early-stage online
Uncompromising security for your PDF editing and eSignature needs
How to fill out regulatory considerations for early-stage

How to fill out regulatory considerations for early-stage
Who needs regulatory considerations for early-stage?
How-to Guide: Regulatory Considerations for Early-Stage Forms
Understanding regulatory considerations
Navigating regulatory frameworks is essential for life sciences companies, especially in their early stages. Compliance is not just an administrative requirement; it’s a fundamental aspect that affects project timelines and funding prospects. Approval from regulatory bodies like the FDA and EMA can greatly influence a startup’s trajectory and credibility in the market.
Failure to adhere to regulatory standards can lead to delays, increased costs, or even project abandonment. Non-compliance not only risks financial backing from investors but may also cause issues with liability and potential legal repercussions. Early-stage ventures must understand these frameworks to ensure that their development processes align with market requirements.
Common regulatory bodies and their roles
Key organizations, such as the FDA in the United States and the EMA in Europe, play a pivotal role in influencing the document creation and submission processes for early-stage forms. Their guidelines dictate the essential components of applications, market authorization, and clinical trial documentation.
Moreover, these bodies require thorough documentation detailing safety and efficacy to assess the potential risks and benefits of new therapies. Knowledge of these requirements is critical not only for compliance but also for establishing credibility with acquirers and investors who look for robust evidential support as a marker of potential exit value.
Identifying necessary forms and documents
Determining the types of forms required for regulatory submission can streamline the process for early-stage ventures. Key documents range from application forms for regulatory approval to safety and efficacy documentation vital for clinical trials. Each document typically follows the stringent guidelines set forth by regulatory bodies.
Additionally, ethical review submissions often take a forefront role, requiring careful attention to detail. In such cases, ensuring that documentation meets the necessary guidelines not only facilitates faster approval but also prevents shortcomings that could derail projects due to inadequate information.
Essential components of each form
Every regulatory form must include specific components that are crucial for a successful submission. These elements often cover detailed project descriptions, methodologies for research, safety data, and the anticipated impact of the proposed studies. Each section should be meticulously crafted to aim for clarity and precision, reflecting the current regulatory requirements.
Furthermore, compliance with specific data formats is non-negotiable. Regulatory agencies tend to have standardized formats that aid in the consistency of submissions. Understanding these formats and incorporating them into the early-stage forms will greatly mitigate the risk of rejection and the ensuing hurdles that can significantly delay project timelines.
Best practices in document creation
Utilizing platforms like pdfFiller can prove beneficial in achieving compliance since it offers features that support document creation aligned with regulatory requirements. For instance, interactive tools allow teams to leverage template options that simplify the drafting of crucial documentation without compromising on detail or accuracy.
Additionally, ensuring accuracy and clarity in regulatory documents is paramount. This can be achieved through systematic reviews and by employing best practices in writing clear and concise content. Regular data integrity checks, combined with user compliance mechanisms within the platform, enhance the trustworthiness of submitted forms and minimize risks related to non-compliance.
Managing changes and updates
Understanding the lifecycle of regulatory forms is vital. Regulations can evolve, necessitating updates to previously submitted forms. This is where a document management solution like pdfFiller shines, offering users the tools they need to keep their documents current and in compliance with the latest guidelines.
Real-time collaboration tools enable team members to handle revisions efficiently, thus ensuring that everyone is aligned with the latest changes. Best practices in version control should also be emphasized, allowing teams to track edits and maintain a coherent document history without confusion.
Collaborative compliance strategies
Engaging stakeholders throughout the compliance process is crucial. Identifying key players such as investors, regulatory advisors, and legal counsel can significantly improve the quality and compliance of submissions. Platforms like pdfFiller foster collaboration by allowing multiple users to contribute to a document while tracking changes in real-time.
Furthermore, organizing workshops or training sessions can elevate team understanding of regulatory requirements. Providing ongoing support and resources empowers teams to navigate complex procedures more proficiently. This includes familiarizing them with tools that can streamline document management, like pdfFiller's extensive features.
Strategies for submission and follow-up
Understanding the electronic submission process is crucial for timely and successful applications for regulatory forms. Knowing the requirements for electronic submissions, including file formats and the use of e-signatures, can expedite approval processes. With pdfFiller, teams can utilize secure document-sharing features that ensure confidentiality and streamline access.
Post-submission, teams should adopt best practices for tracking submitted documents and maintaining open communication with regulatory bodies. Establishing a system for managing correspondence can help anticipate potential questions or issues, facilitating a smoother overall process.
Future trends in regulatory compliance
As the regulatory landscape continually evolves, early-stage ventures must remain vigilant about emerging changes that could impact their documentation strategies. Keeping an eye on anticipated reforms helps teams prepare in advance, ensuring compliance and avoiding potential setbacks when it comes to new regulations.
Moreover, leveraging technology can enhance compliance efforts. Innovations in document management and compliance monitoring tools expand capabilities for life sciences companies, making it easier to adapt to shifting requirements. pdfFiller stands at the forefront of this transition, providing solutions that offer users flexibility in managing their documentation amidst evolving regulations.
Case studies and practical applications
Studying success stories of compliance in early-stage ventures can reveal valuable insights into effective strategies and best practices. Many life sciences companies have successfully navigated regulatory hurdles by adhering to meticulous documentation practices and learning from industry best practices. These exemplary cases serve as benchmarks for new startups.
Conversely, analyzing lessons learned from non-compliance issues underscores areas needing improvement. Instances of regulatory failures often highlight gaps in documentation or misunderstandings of requirements, showcasing how adherence to regulations can determine the viability and potential success or failure of projects in development.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send regulatory considerations for early-stage to be eSigned by others?
How can I edit regulatory considerations for early-stage on a smartphone?
How do I edit regulatory considerations for early-stage on an Android device?
What is regulatory considerations for early-stage?
Who is required to file regulatory considerations for early-stage?
How to fill out regulatory considerations for early-stage?
What is the purpose of regulatory considerations for early-stage?
What information must be reported on regulatory considerations for early-stage?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















