Get the free BCPP Preparatory Product Registration is Live
Get, Create, Make and Sign bcpp preparatory product registration



Editing bcpp preparatory product registration online
Uncompromising security for your PDF editing and eSignature needs
How to fill out bcpp preparatory product registration

How to fill out bcpp preparatory product registration
Who needs bcpp preparatory product registration?
BCPP Preparatory Product Registration Form: A Comprehensive Guide
Understanding the BCPP preparatory product registration process
BCPP registration plays a crucial role in the lifecycle of products intended for clinical applications. This preparatory process ensures that products meet necessary standards for safety, efficacy, and quality before they enter the market. Stakeholders such as manufacturers, regulatory bodies, and clinical practitioners are integral to the registration process, as they each hold a vested interest in compliance.
The importance of BCPP registration cannot be overstated. It serves as a framework guiding products from development to public distribution, ensuring all relevant regulations are met. Companies must navigate this process to enhance trust and legitimacy in their offerings, supporting sustained market interest.
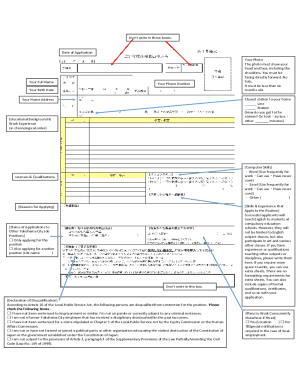
Essential components of the BCPP preparatory product registration form
Completing the BCPP preparatory product registration form requires detailed information that captures the essence of the product and its manufacturing background. Accurate product identification details, including naming conventions and classification, are fundamental. Moreover, the form must request manufacturer and distributor information to maintain traceability and accountability throughout the distribution chain.
Safety and quality assurance documentation is another crucial aspect of the submission. Supporting documentation must include a Certificate of Analysis (CoA) alongside distinct labeling and packaging specifications that reflect marketing standards. Including comprehensive research and development data informs regulatory bodies about the methodologies and validations that back the product's claims.
It is common for applicants to have questions regarding documentation requirements. Clarifications regarding the need for certain certifications or approvals can help mitigate errors during submission. Frequently asked questions often address common missteps that lead to delays or additional requests for information.
Step-by-step guide to completing the BCPP preparatory product registration form
The preparation phase for the BCPP preparatory product registration form centers on gathering all necessary documents. Start by compiling a checklist that includes every piece of documentation you need, as this will facilitate a smoother submission process. Familiarize yourself with formatting requirements, especially for electronic submissions since compliance with these specifications can influence processing times.
When it comes to filling out the registration form, meticulously input product information in the designated fields, ensuring alignment with previous sections. Follow this with detailed manufacturer information, confirming that each entry is accurate and matches the supporting documents. Finally, meticulously detail the supporting documentation, ensuring that all references are correctly noted.
Before submitting, take the time to review your submission thoroughly. Double-check for accuracy and completeness, as ensuring error-free documentation is vital. Minor mistakes can lead to significant delays or complications, hindering the product's market entry.
Interactive tools for efficient registration
Using tools such as pdfFiller enhances the experience of completing the BCPP preparatory product registration form. The platform offers advanced online PDF editing features, enabling users to modify forms directly without additional software. Collaboration tools facilitate team input, ensuring that all stakeholders can contribute to the documentation process efficiently.
The eSignature capabilities within pdfFiller present another level of convenience. Signatures can be added electronically, expediting the approval process while maintaining legal compliance. Utilizing pdfFiller's document management system, users can securely store and organize their forms on a cloud-based platform, simplifying access and retrieval when necessary.
Post-submission actions and best practices
After submitting the BCPP preparatory product registration form, understanding what to expect is essential. A typical timeline for registration approval can vary widely, often ranging from weeks to months, depending on the regulatory body involved. Be prepared for potential follow-up inquiries, as agencies may seek further clarification or documentation before moving forward with the registration process.
Compliance doesn’t stop once registration is complete. Post-registration, conduct routine checks on product performance and adherence to regulatory standards. Remaining proactive about compliance, including timely updates and renewals, ensures that your product remains in good standing. This diligence not only maintains market presence but also builds credibility within the clinical application series.
Troubleshooting common issues during the registration process
Navigating the BCPP preparatory product registration form can pose challenges for many applicants. Common pitfalls include incomplete submissions, lack of required documentation, and incorrect information, all of which can delay or derail the registration process. It’s critical to take proactive measures by educating your team on best practices and what the regulatory agencies specifically require.
If errors disrupt your registration flow, having a plan in place to resolve these challenges efficiently is essential. Engaging with resources dedicated to regulatory inquiries can provide clarity and guide you through complex situations. Additionally, utilizing support from pdfFiller can streamline document-related issues, ensuring a smoother experience.
Case studies: Successful BCPP registrations
Analyzing successful BCPP registrations offers valuable insights into effective strategies. Companies that approached the registration process with thorough preparation and proactive engagement with regulatory bodies often saw smoother outcomes. By employing a structured approach to documentation, including well-organized research and development data, these companies minimized delays and streamlined their approval timelines.
Lessons learned emphasize the importance of building strong relationships with regulatory agencies while seeking feedback throughout the registration phase. Engaging regularly with stakeholders can provide critical insights for future registrations, making the process more efficient with each iteration.
Continuous improvement for future registrations
Integrating a feedback mechanism is crucial for enhancing the BCPP preparatory product registration process. Gathering insights from applicants about their experiences using the pdfFiller platform can reveal areas for improvement. By actively soliciting user experiences and recommendations, developers can refine the registration forms and associated processes, ensuring that they align with real user needs.
Implementing these enhancements entails an iterative process where suggestions are analyzed and prioritized for development. As the regulatory landscape evolves, adapting the registration process to maintain relevance and efficiency benefits all stakeholders involved. Continuous improvement not only optimizes each registration but also contributes positively to the entire clinical application series.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get bcpp preparatory product registration?
Can I create an electronic signature for the bcpp preparatory product registration in Chrome?
How do I complete bcpp preparatory product registration on an iOS device?
What is bcpp preparatory product registration?
Who is required to file bcpp preparatory product registration?
How to fill out bcpp preparatory product registration?
What is the purpose of bcpp preparatory product registration?
What information must be reported on bcpp preparatory product registration?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.