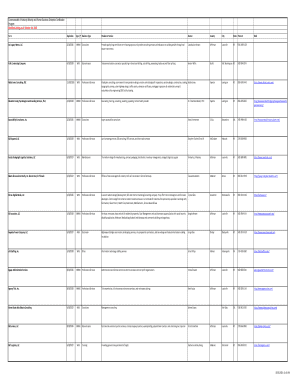
Get the free Re-Bidding of Drugs and Medicines 2021 charged to Trust ...
Get, Create, Make and Sign re-bidding of drugs and



How to edit re-bidding of drugs and online
Uncompromising security for your PDF editing and eSignature needs
How to fill out re-bidding of drugs and

How to fill out re-bidding of drugs and
Who needs re-bidding of drugs and?
Re-bidding of Drugs and Form: A Comprehensive Guide
Understanding the re-bidding process for pharmaceutical supplies
Re-bidding of drugs plays a crucial role in the pharmaceutical industry, driven by the need for competitive pricing and compliance with regulatory frameworks. This process often occurs when contracts with existing suppliers come to an end or when market conditions change, necessitating reassessment of suppliers. By re-bidding, organizations can leverage market competition, ensuring they obtain the best possible prices and product quality.
The importance of re-bidding cannot be understated; it fosters cost efficiency, promotes compliance with regulations, and can reflect evolving healthcare needs. Key stakeholders involved in the re-bidding process typically include procurement teams, pharmaceutical suppliers, regulatory bodies, and sometimes, end-users, such as healthcare practitioners and their patients. These groups collaborate to ensure that the requirements of the new supply contracts align with public health needs.
Preparing for re-bidding: essential steps
Preparing for a re-bidding process requires a structured approach. The first step is to analyze previous bids, which involves conducting a detailed review of past procurement practices. Understanding the strengths and weaknesses of prior bids can illuminate areas for improvement and set a baseline for future proposals. Organizations should review the performance of past contracts, taking note of suppliers' reliability and the quality of drugs provided.
Next, it’s vital to define clear and precise requirements. Setting specifications for drugs and services is essential, as this helps ensure compliance with applicable regulations and quality standards. Organizations should also engage stakeholders in determining these requirements to reflect the actual needs of healthcare providers and patients.
Lastly, budgeting for the re-bid is crucial. This involves financial forecasting and considering funding options, while also performing cost-effectiveness analyses. Organizations must ensure they allocate sufficient resources to support the re-bidding process and align their financial resources with strategic goals.
Drafting the invitation to bid (ITB)
Drafting a compelling Invitation to Bid (ITB) is pivotal for a successful re-bidding process. An effective ITB should include several key elements, starting with a clear scope of work and deliverables. Potential suppliers need to understand the expectations and requirements to submit comprehensive bids.
Additionally, the ITB should outline a timeline for the process, including submission deadlines and evaluation periods. Specifying the evaluation criteria within the ITB helps ensure that bidders know how their submissions will be assessed, allowing for a more transparent selection process.
Selecting the right platform for distributing the ITB can further enhance outreach. Digital tools can be leveraged for wider reach, allowing for a more diverse pool of bidders. Transparency throughout the bidding process is vital in maintaining trust among all stakeholders.
Evaluating bids: making informed decisions
Once the bids have been submitted, the evaluation phase begins. Key criteria for assessing submissions typically includes price, quality of products, and supplier reliability. Regulatory compliance cannot be overlooked, as it ensures that the selected medications meet all necessary guidelines.
Utilizing structured tools for bid analysis, such as bid comparison matrices, can streamline this process. These tools help by providing a clear visual overview of how each supplier measures up against the established criteria. More advanced digital solutions can also facilitate analysis, reducing the room for error and ensuring a fair evaluation.
Awarding contracts: best practices
Once the evaluations are complete, it’s time to award the contracts. Communication with bidders about the decision is essential, whether or not they are the chosen suppliers. Providing constructive feedback to unsuccessful bidders can enhance relationships for future opportunities.
In some cases, disputes or appeals may arise post-award. Establishing a clear process for handling these situations, including timelines and a review mechanism, helps mitigate potential conflicts. Setting clear expectations for contract management ultimately leads to better supplier relationships and smoother operations.
Managing drugs post-bid award
Effective management of drug supplies after the award is critical. This includes creating a thorough onboarding process for new suppliers to ensure they understand contractual obligations and performance expectations. Continuous monitoring and quality assurance during this phase are equally important.
Establishing key performance indicators (KPIs) to measure supplier performance can support ongoing assessment. Regular audits and compliance checks are also essential in maintaining quality standards and ensuring suppliers adhere to contractual terms. This continuous feedback loop helps develop stronger partnerships and improves overall procurement strategies.
Innovations in re-bidding processes
Technological advancements are reshaping the landscape of re-bidding in the pharmaceutical sector. Leveraging technology for efficiency can simplify document management, making processes more streamlined and less time-consuming. Solutions like pdfFiller allow users to edit PDF submissions easily, eSign contracts digitally, and collaborate on documents in real-time.
Furthermore, the shift towards digital platforms is also fostering a more transparent bidding environment. The emerging trends in drug procurement are significantly influenced by technological integration, which can lead to enhanced efficiency in the re-bidding process.
Case studies: successful re-bidding examples
Examining real-world examples of successful re-bidding strategies provides valuable insights. For instance, consider a case where a regional healthcare system implemented a new procurement strategy, leading to a significant reduction in costs while improving the quality of medications supplied. Lessons learned from such cases highlight the importance of adaptability and collaboration among stakeholders to achieve procurement success.
These insights can guide organizations through their own re-bidding efforts, providing transferable strategies and tools that have proven effective in different sectors, ultimately ensuring that healthcare providers can meet their objectives efficiently.
Interactive tools for your re-bidding journey
Utilizing interactive tools can greatly aid the re-bidding journey. Document templates available on pdfFiller can facilitate the re-bidding process, providing specific forms for drug procurement and compliance. These templates offer a structured foundation from which to build effective ITBs and contracts.
Additionally, employing interactive checklists aids in ensuring that all necessary steps are followed throughout the process. Digital tools enhance collaboration and streamline document edits and submissions, allowing teams to remain coordinated and engaged despite remote work environments.
Ensuring compliance and ethical standards
Adhering to compliance and ethical standards remains paramount throughout the re-bidding of drugs. Organizations must prioritize transparency and accountability to maintain trust with stakeholders and avoid potential legal implications from non-compliance. By integrating ethical considerations into their procurement processes, organizations ensure that their sourcing practices reflect society’s standards.
Implementing best practices for transparency communicates a commitment to ethical procurement and fosters better relationships with suppliers. This results in a more robust reputation and the possibility for future collaborations that are beneficial for all parties involved.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify re-bidding of drugs and without leaving Google Drive?
Where do I find re-bidding of drugs and?
How do I fill out re-bidding of drugs and on an Android device?
What is re-bidding of drugs and?
Who is required to file re-bidding of drugs and?
How to fill out re-bidding of drugs and?
What is the purpose of re-bidding of drugs and?
What information must be reported on re-bidding of drugs and?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.