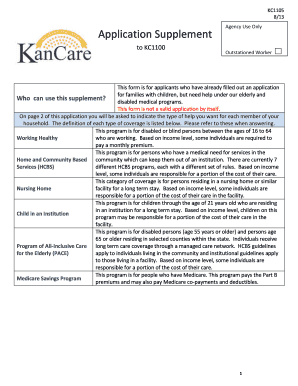
Get the free Human Participants Research - Informed Consent Checklist
Show details
Informed Consent for Human Participants CHECKLIST FOR RESEARCHERS This checklist is intended to assist researchers with the kind of information that may be required in developing written Informed
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign human participants research

Edit your human participants research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your human participants research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit human participants research online
Follow the steps below to use a professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit human participants research. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out human participants research

How to fill out human participants research?
01
Start by clearly defining the research objectives and hypotheses. This will ensure that the data collected from human participants aligns with the goals of the study.
02
Obtain the necessary ethical approval and informed consent forms from the relevant institutional review board or ethics committee. This step is crucial to protect the rights and well-being of the participants.
03
Develop a comprehensive and well-structured questionnaire or interview guide that will gather the required data for the research. Ensure that the questions are clear, unbiased, and relevant to the research objectives.
04
Recruit a diverse and representative sample of participants that aligns with the target population of the study. Use appropriate methods such as random sampling or stratified sampling to ensure the sample is unbiased.
05
Conduct the research study either through in-person interviews, online surveys, focus groups, or any other appropriate method. Make sure to provide clear instructions to the participants and maintain their privacy and confidentiality throughout the process.
06
Collect and record the data accurately and systematically. This may involve using specialized software or tools to organize and analyze the data.
07
Analyze the collected data using appropriate statistical or qualitative analysis techniques, depending on the research design and objectives.
08
Interpret the results of the analysis and draw meaningful conclusions that address the research questions or hypotheses.
09
Communicate the findings of the research through academic journals, conferences, reports, or any other relevant medium.
10
Finally, review and reflect on the research process and outcomes. Identify any limitations or areas for improvement and consider the implications for future research.
Who needs human participants research?
01
Researchers in various scientific disciplines, such as psychology, sociology, medicine, and education, often need human participants research to understand human behavior, attitudes, and experiences.
02
Organizations and institutions seeking to develop evidence-based policies and interventions rely on human participants research to inform their decision-making processes.
03
Funding agencies, both governmental and private, often require human participants research to assess the impact and effectiveness of programs or initiatives they support.
04
Students and academics pursuing higher education degrees may need to conduct human participants research as part of their coursework or thesis projects.
05
Professionals in market research, consumer behavior, and usability testing often rely on human participants research to gather insights and make informed decisions about product development, marketing strategies, or user experience improvements.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my human participants research directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign human participants research and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
How can I send human participants research for eSignature?
To distribute your human participants research, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
How do I complete human participants research online?
Filling out and eSigning human participants research is now simple. The solution allows you to change and reorganize PDF text, add fillable fields, and eSign the document. Start a free trial of pdfFiller, the best document editing solution.
What is human participants research?
Human participants research is a study that involves individuals as subjects, conducted to advance scientific knowledge.
Who is required to file human participants research?
Researchers or institutions conducting human participants research are required to file the necessary paperwork and comply with regulations.
How to fill out human participants research?
To fill out human participants research, researchers need to provide detailed information about the study design, participant demographics, informed consent process, and risk assessment.
What is the purpose of human participants research?
The purpose of human participants research is to gather new information, improve understanding of specific topics, or develop new treatments or interventions.
What information must be reported on human participants research?
Information that must be reported on human participants research includes study protocols, consent forms, participant data, adverse events, and potential risks and benefits.
Fill out your human participants research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Human Participants Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.