Get the free Regulatory File Essential Documents
Show details
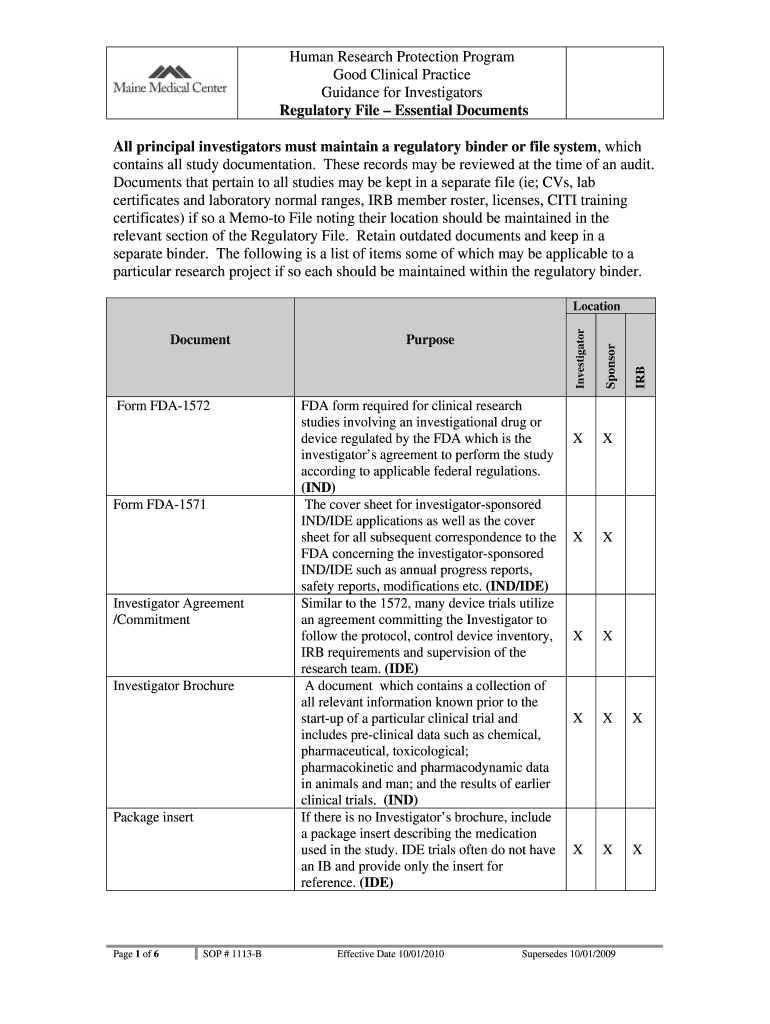
Human Research Protection Program Good Clinical Practice Guidance for Investigators Regulatory File Essential Documents All principal investigators must maintain a regulatory binder or file system,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign regulatory file essential documents

Edit your regulatory file essential documents form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your regulatory file essential documents form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing regulatory file essential documents online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit regulatory file essential documents. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out regulatory file essential documents

How to fill out regulatory file essential documents:
01
Read the regulations: Start by familiarizing yourself with the specific regulations and requirements related to the industry or field for which you are creating the regulatory file. This will help you understand what documents are necessary and what information needs to be included.
02
Gather relevant information: Collect all the necessary information and data that needs to be included in the regulatory file. This may include details about the product or service, safety information, manufacturing processes, financial records, and any other relevant documentation.
03
Organize the documents: Create a structure or organization system for the regulatory file. This could involve creating folders or sections for different types of documents or using a digital filing system. Ensure that the documents are easily accessible and properly labeled for quick reference.
04
Complete the necessary forms: Fill out any required forms or templates that are part of the regulatory file. These forms may need to be filled out with specific information and signed by relevant parties. Follow the instructions carefully and provide accurate information.
05
Review and validate the information: Double-check all the documents and information included in the regulatory file for accuracy and completeness. Make sure that all necessary documents are included and that the information provided is up to date and valid.
06
Seek expert advice if needed: If you are unsure about any aspect of filling out the regulatory file or if you need clarification on certain requirements, consider seeking expert advice. Reach out to regulatory professionals or consult with knowledgeable individuals in your industry.
Who needs regulatory file essential documents?
01
Companies in regulated industries: Businesses operating in industries such as pharmaceuticals, healthcare, food and beverages, and financial services are often required to maintain regulatory file essential documents. These documents serve as evidence of compliance with relevant regulations and may need to be submitted to regulatory authorities.
02
Government agencies: Regulatory file essential documents may be required by government agencies responsible for monitoring and enforcing regulations in specific industries. These agencies may review the documents to ensure compliance and assess the safety and quality standards of products and services.
03
Auditors and inspectors: Independent auditors and inspectors may request access to regulatory file essential documents as part of their assessments. These professionals evaluate company practices, compliance, and adherence to regulatory guidelines to identify any non-compliance issues or areas for improvement.
In summary, filling out regulatory file essential documents involves understanding the regulations, gathering and organizing necessary information, completing required forms, reviewing for accuracy, and seeking expert advice if needed. These documents are necessary for companies in regulated industries, government agencies, and auditors/inspectors to ensure compliance and assess the safety and quality standards.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is regulatory file essential documents?
Regulatory file essential documents are the necessary paperwork and information required by regulatory bodies to ensure compliance with laws and regulations.
Who is required to file regulatory file essential documents?
Any individual, organization, or company that is subject to regulatory requirements must file regulatory file essential documents.
How to fill out regulatory file essential documents?
Regulatory file essential documents can be filled out by providing accurate and detailed information as requested by the regulatory body.
What is the purpose of regulatory file essential documents?
The purpose of regulatory file essential documents is to demonstrate compliance with laws and regulations, as well as to provide transparency and accountability.
What information must be reported on regulatory file essential documents?
The information required on regulatory file essential documents may vary depending on the specific regulations, but typically includes financial data, operational details, and compliance information.
Where do I find regulatory file essential documents?
The pdfFiller premium subscription gives you access to a large library of fillable forms (over 25 million fillable templates) that you can download, fill out, print, and sign. In the library, you'll have no problem discovering state-specific regulatory file essential documents and other forms. Find the template you want and tweak it with powerful editing tools.
How do I complete regulatory file essential documents online?
pdfFiller has made filling out and eSigning regulatory file essential documents easy. The solution is equipped with a set of features that enable you to edit and rearrange PDF content, add fillable fields, and eSign the document. Start a free trial to explore all the capabilities of pdfFiller, the ultimate document editing solution.
Can I create an electronic signature for signing my regulatory file essential documents in Gmail?
Upload, type, or draw a signature in Gmail with the help of pdfFiller’s add-on. pdfFiller enables you to eSign your regulatory file essential documents and other documents right in your inbox. Register your account in order to save signed documents and your personal signatures.
Fill out your regulatory file essential documents online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Regulatory File Essential Documents is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















