Get the free Successful Management of FDA IDE
Show details
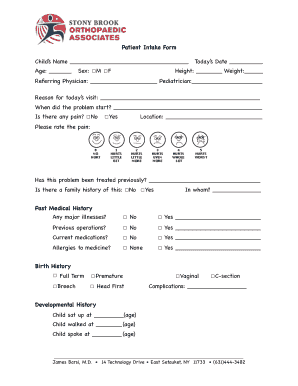
Successful Management of FDA IDE Studies Investigational Device Exemption (IDE)Melissa Born CR Monitoring Manager Anna Stravinsky Sr. CR Monitoring Specialist Office of Clinical Research March 20,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign successful management of fda

Edit your successful management of fda form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your successful management of fda form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit successful management of fda online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit successful management of fda. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out successful management of fda

How to fill out successful management of FDA:
01
Establish clear goals and objectives: Start by defining what you want to achieve with the management of FDA. Identify the specific areas or processes that need improvement and set realistic and measurable goals.
02
Develop a comprehensive plan: Create a detailed plan that outlines the steps and strategies to be implemented for effective FDA management. This should include timelines, resources required, and a clear delegation of responsibilities.
03
Stay updated with regulations and guidelines: It is crucial to stay informed about the latest regulations, guidelines, and standards set by the FDA. Regularly review and update your knowledge to ensure compliance with the necessary requirements.
04
Utilize appropriate technology and tools: Invest in reliable software and tools that can streamline and automate various processes related to FDA management. These can include document management systems, data analytics software, and electronic submission platforms.
05
Implement proper training and education: Train your staff on FDA regulations, quality control processes, and good manufacturing practices. Provide ongoing education and ensure that everyone involved understands their roles and responsibilities in maintaining FDA compliance.
06
Conduct regular audits and inspections: Perform internal audits to assess the effectiveness of your FDA management processes. Additionally, be prepared for external inspections or audits by the FDA to demonstrate your compliance and commitment to quality.
07
Foster a culture of quality and compliance: Instill a culture within your organization that values quality, compliance, and continuous improvement. Encourage open communication, active participation, and accountability at all levels.
Who needs successful management of FDA:
01
Pharmaceutical companies: Pharmaceutical companies must adhere to FDA regulations to ensure the safety and efficacy of their products. Effective FDA management helps them navigate the complex regulatory landscape, maintain compliance, and avoid costly penalties.
02
Medical device manufacturers: Similar to pharmaceutical companies, medical device manufacturers are subject to FDA regulations. Successful FDA management ensures that their products meet the necessary safety and quality standards before they are brought to market.
03
Food and beverage industry: Businesses involved in the production and distribution of food and beverages must comply with FDA regulations to ensure the safety and integrity of their products. Proper FDA management is essential for maintaining consumer trust and avoiding potential recalls or legal issues.
04
Clinical research organizations (CROs) and research institutions: CROs and research institutions conducting clinical trials need effective FDA management to ensure compliance with the required protocols, data integrity, and ethical considerations. This management is vital for obtaining FDA approval for new drugs or medical devices.
05
Regulatory affairs professionals: Professionals working in regulatory affairs play a crucial role in managing FDA compliance. They are responsible for interpreting regulatory requirements, preparing documentation for FDA submissions, and ensuring ongoing compliance with FDA regulations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is successful management of fda?
Successful management of FDA involves effectively overseeing and complying with regulations set forth by the Food and Drug Administration.
Who is required to file successful management of fda?
Companies and individuals involved in the manufacturing, distribution, and sale of FDA-regulated products are required to file successful management reports with the FDA.
How to fill out successful management of fda?
To fill out a successful management report for the FDA, companies and individuals must provide accurate and detailed information regarding their products, facilities, and compliance measures.
What is the purpose of successful management of fda?
The purpose of successful management reporting to the FDA is to ensure that regulated products meet safety and quality standards, and that companies are in compliance with applicable regulations.
What information must be reported on successful management of fda?
Information that must be reported on a successful management report to the FDA includes product details, facility information, quality control measures, and any non-compliance issues.
How do I execute successful management of fda online?
pdfFiller has made it simple to fill out and eSign successful management of fda. The application has capabilities that allow you to modify and rearrange PDF content, add fillable fields, and eSign the document. Begin a free trial to discover all of the features of pdfFiller, the best document editing solution.
Can I sign the successful management of fda electronically in Chrome?
Yes. With pdfFiller for Chrome, you can eSign documents and utilize the PDF editor all in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a handwritten signature image. You may eSign your successful management of fda in seconds.
How can I edit successful management of fda on a smartphone?
The pdfFiller apps for iOS and Android smartphones are available in the Apple Store and Google Play Store. You may also get the program at https://edit-pdf-ios-android.pdffiller.com/. Open the web app, sign in, and start editing successful management of fda.
Fill out your successful management of fda online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Successful Management Of Fda is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.