Get the free Large observational study to UNderstand the Global impact of Severe Acute respirator...
Show details
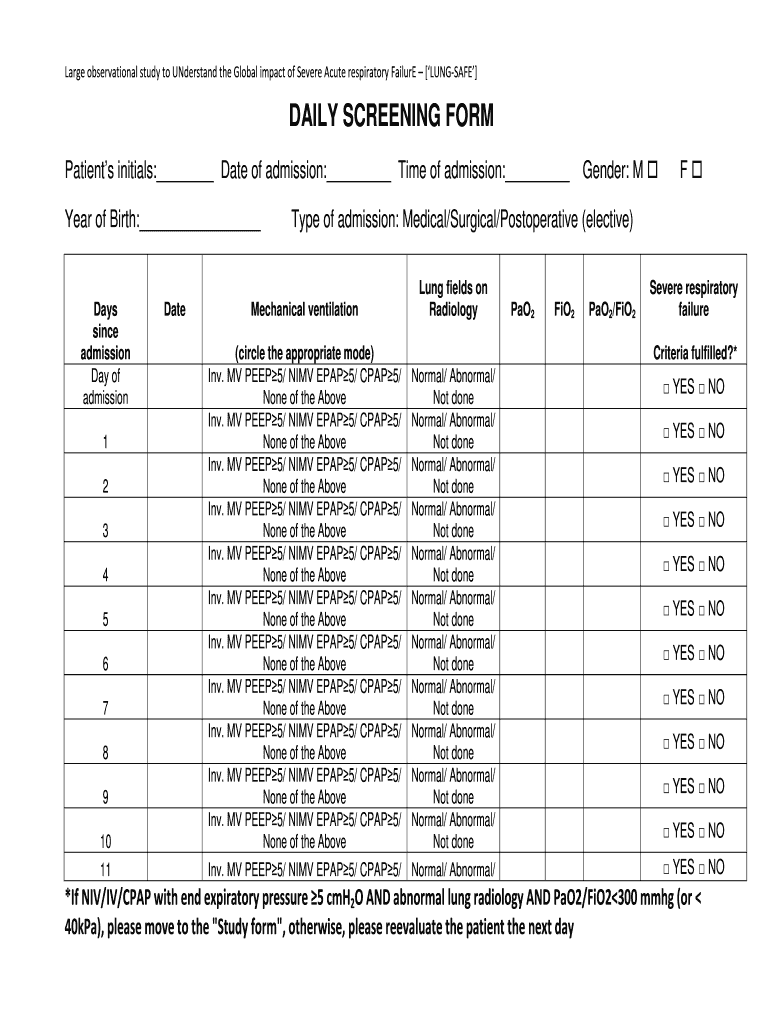
Large observational study to Understand the Global impact of Severe Acute respiratory Failure LUNGS AFE DAILY SCREENING FORM Patients initials: Date of admission: Time of admission: Year of Birth:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign large observational study to

Edit your large observational study to form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your large observational study to form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing large observational study to online
To use our professional PDF editor, follow these steps:
1
Check your account. In case you're new, it's time to start your free trial.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit large observational study to. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
The use of pdfFiller makes dealing with documents straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out large observational study to

Point by point, here is how to fill out a large observational study:
01
Begin by clearly defining the objectives and research questions of your study. This will help guide your data collection and analysis.
02
When selecting the study population, ensure it is representative of the target population you wish to study. This will increase the external validity of your findings.
03
Develop a detailed study protocol outlining the methodology, data collection procedures, and ethical considerations. This protocol serves as a roadmap for conducting the study.
04
Choose appropriate data collection methods, whether it be surveys, interviews, medical records review, or direct observations. Ensure that the data collected aligns with your research objectives.
05
Implement a robust data management system to organize and store the collected data securely. This will facilitate data analysis and future replication of the study.
06
Analyze the collected data using appropriate statistical techniques and qualitative analysis methods if applicable. This will help answer your research questions and draw meaningful conclusions.
07
Interpret the results and discuss their implications in the context of existing literature. Analyze the strengths and limitations of your study and propose recommendations for future research or interventions.
08
Finally, disseminate your findings through academic publications, conferences, or other appropriate channels. This will contribute to the scientific knowledge base and potentially inform policy or practice.
As for who needs a large observational study, it could be beneficial for various stakeholders:
01
Researchers and scientists who aim to investigate the relationship between variables or explore phenomena within a specific population.
02
Healthcare professionals and policymakers who need evidence-based data to guide decision-making and improve patient outcomes.
03
Pharmaceutical companies or medical device manufacturers seeking to evaluate the safety and effectiveness of their products in real-world settings.
04
Public health organizations and government agencies interested in monitoring population health trends and designing targeted interventions.
05
Insurance companies and healthcare providers aiming to identify risk factors, improve care delivery, and optimize resource allocation.
In summary, a large observational study can be useful to numerous individuals and organizations involved in research, healthcare, policy-making, and public health initiatives.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit large observational study to online?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your large observational study to to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
How do I edit large observational study to on an Android device?
You can make any changes to PDF files, like large observational study to, with the help of the pdfFiller Android app. Edit, sign, and send documents right from your phone or tablet. You can use the app to make document management easier wherever you are.
How do I complete large observational study to on an Android device?
Use the pdfFiller mobile app to complete your large observational study to on an Android device. The application makes it possible to perform all needed document management manipulations, like adding, editing, and removing text, signing, annotating, and more. All you need is your smartphone and an internet connection.
What is large observational study to?
Large observational study is typically conducted to study the real-world impact of a particular treatment or intervention.
Who is required to file large observational study to?
Researchers, healthcare professionals, or institutions conducting the study may be required to file large observational study.
How to fill out large observational study to?
The study must be filled out accurately, including all relevant data and information about the study design, participants, outcomes, and conclusions.
What is the purpose of large observational study to?
The purpose of large observational study is to gather data on the effectiveness and safety of a treatment or intervention in real-world settings.
What information must be reported on large observational study to?
Information such as study design, participant demographics, outcomes, adverse events, and conclusions must be reported on large observational study.
Fill out your large observational study to online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Large Observational Study To is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















