Get the free Disclosure of Adverse Events in the United States and ... - NCBI - mitsstools
Show details
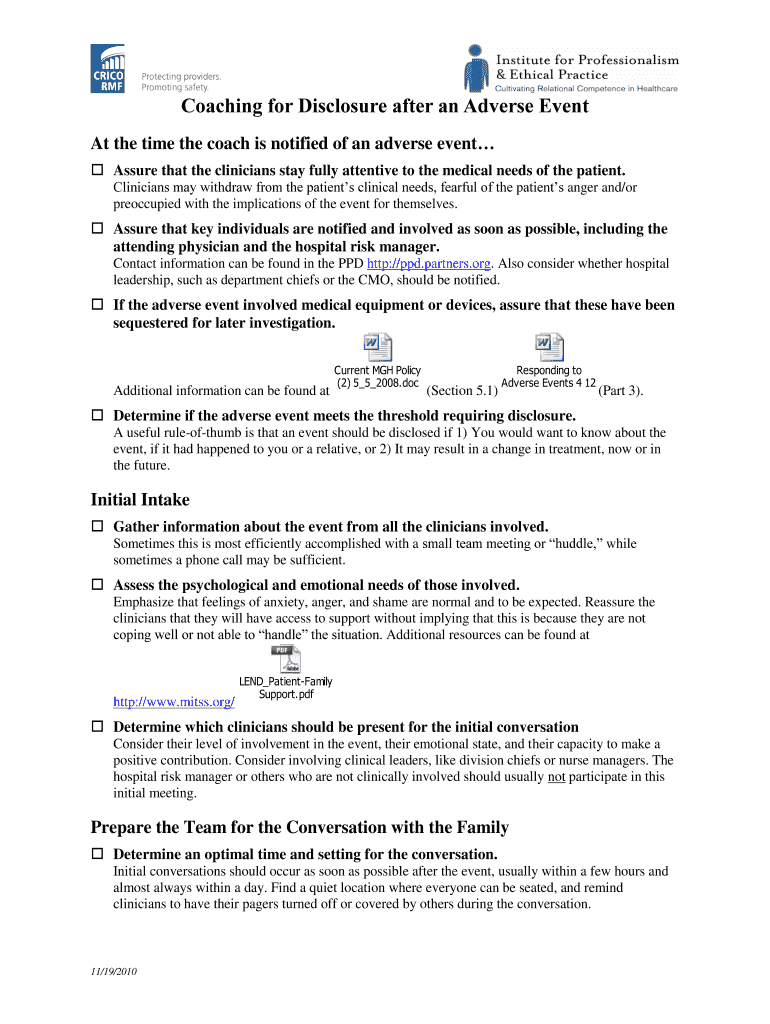
Coaching for Disclosure after an Adverse Event At the time the coach is notified of an adverse event Assure that the clinicians stay fully attentive to the medical needs of the patient. Clinicians
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign disclosure of adverse events

Edit your disclosure of adverse events form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your disclosure of adverse events form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit disclosure of adverse events online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit disclosure of adverse events. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out disclosure of adverse events

How to fill out disclosure of adverse events:
01
Gather all relevant information: Before filling out the disclosure, gather all necessary information related to the adverse event. This may include the date and time of the event, a description of what occurred, any relevant documents or reports, and the names of any individuals involved.
02
Use the appropriate form: Each organization or institution may have its own specific form for disclosing adverse events. Locate the correct form and ensure you are using the most up-to-date version. If you are uncertain about which form to use, consult with your supervisor or the appropriate department.
03
Provide accurate and detailed information: When filling out the disclosure form, it is important to be thorough and provide accurate information about the adverse event. Include specific details about what happened, any contributing factors, and any actions that were taken immediately following the event. Avoid making assumptions or speculations; stick to the facts as you know them.
04
Use clear and concise language: When describing the adverse event, use clear and concise language that can be easily understood by others. Avoid medical jargon or technical terms that may be unfamiliar to those reviewing the disclosure. If necessary, provide explanations or definitions for any specialized terminology used.
05
Be transparent and honest: Honesty and transparency are crucial when filling out a disclosure of adverse events. Provide a truthful account of what happened, including any errors or mistakes that may have occurred. If you are unsure about certain details, indicate that you are uncertain rather than providing inaccurate information.
06
Follow any additional instructions: Some organizations may have specific guidelines or instructions for filling out the disclosure form. Make sure to read and follow these instructions carefully. This may include submitting the form to a particular department, attaching supporting documents, or notifying specific individuals about the disclosure.
Who needs disclosure of adverse events:
01
Healthcare providers: Healthcare providers, including doctors, nurses, and other medical professionals, need to disclose adverse events that occur during patient care. This includes incidents such as medication errors, surgical complications, or any other adverse outcome related to a patient's treatment.
02
Pharmaceutical companies: Pharmaceutical companies are required to disclose any adverse events or side effects associated with their drugs or medications. This helps to ensure the safety and efficacy of the products they produce.
03
Research institutions: Research institutions conducting clinical trials or experiments involving human subjects must disclose any adverse events that occur during the course of the study. This ensures that participants are aware of any potential risks and that the necessary actions are taken to protect their well-being.
04
Regulatory agencies: Regulatory agencies, such as the Food and Drug Administration (FDA) in the United States, rely on disclosure of adverse events to monitor the safety and effectiveness of healthcare products and services. These agencies use the information collected to make informed decisions and take appropriate actions.
05
Patients and their families: Patients and their families have the right to be informed about any adverse events that occur in their healthcare journey. This allows them to make informed decisions about their treatment options and provides an opportunity for open communication between patients and healthcare providers.
In summary, filling out a disclosure of adverse events requires gathering accurate information, using the correct form, providing clear and honest descriptions, and following any specific instructions. Healthcare providers, pharmaceutical companies, research institutions, regulatory agencies, and patients and their families are among those who need to be aware of adverse events and participate in the disclosure process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send disclosure of adverse events to be eSigned by others?
When you're ready to share your disclosure of adverse events, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
How do I edit disclosure of adverse events in Chrome?
disclosure of adverse events can be edited, filled out, and signed with the pdfFiller Google Chrome Extension. You can open the editor right from a Google search page with just one click. Fillable documents can be done on any web-connected device without leaving Chrome.
How do I edit disclosure of adverse events straight from my smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing disclosure of adverse events right away.
What is disclosure of adverse events?
Disclosure of adverse events is the process of reporting any harmful events or incidents that occur during healthcare treatment.
Who is required to file disclosure of adverse events?
Healthcare providers and facilities are required to file disclosure of adverse events.
How to fill out disclosure of adverse events?
Disclosure of adverse events can be filled out by providing detailed information about the event, including when it occurred, what caused it, and any resulting harm.
What is the purpose of disclosure of adverse events?
The purpose of disclosure of adverse events is to improve patient safety and transparency in healthcare by identifying and addressing any issues that may have contributed to the adverse event.
What information must be reported on disclosure of adverse events?
Information that must be reported on disclosure of adverse events includes details about the event, the patient involved, any contributing factors, and actions taken to prevent future occurrences.
Fill out your disclosure of adverse events online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Disclosure Of Adverse Events is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















