Regulate Footnote Record For Free




Join the world’s largest companies
Video Review on How to Regulate Footnote Record









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Regulate Footnote Record Feature
The Regulate Footnote Record feature streamlines your documentation process, making it easier to manage and reference footnotes across your projects. This tool is designed for simplicity and efficiency, ensuring that you can focus on your content without worrying about formatting issues.
Key Features
Potential Use Cases and Benefits
Whether you are writing a research paper, preparing a business report, or drafting a novel, the Regulate Footnote Record feature helps you organize your footnotes with ease. It tackles the common issue of lost or misplaced references, allowing you to maintain clarity and professionalism in your work.
Instructions and Help about Regulate Footnote Record For Free
Regulate Footnote Record: simplify online document editing with pdfFiller
Rather than filing your documents manually, discover modern online solutions for all types of paperwork. Nevertheless, most of them are restricted in features or require installing software and take up storage space. Try pdfFiller if you need more than just essential tools and if you need to be able to edit and sign PDF templates everywhere.
pdfFiller is a powerful, web-based document management service with an array of features for editing PDF files. This platform will be perfect for those who often find themselves in need to modify documents in PDF, fill out the form in Word, or convert a scanned image to editable format. Make all the documents fillable, submit applications, complete forms, sign contracts, and so on.
Go
Navigate to the pdfFiller website to begin working with your documents paper-free. Pick any template on your device and upload it to the editing tool. You'll
you will be able to easily access any editing tool you need in just one click.
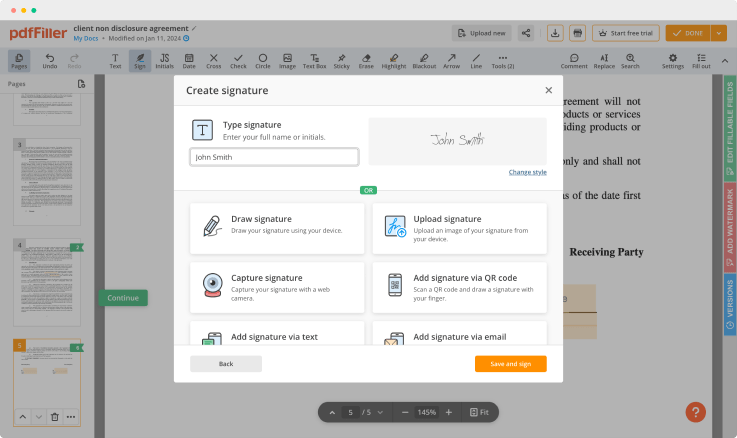
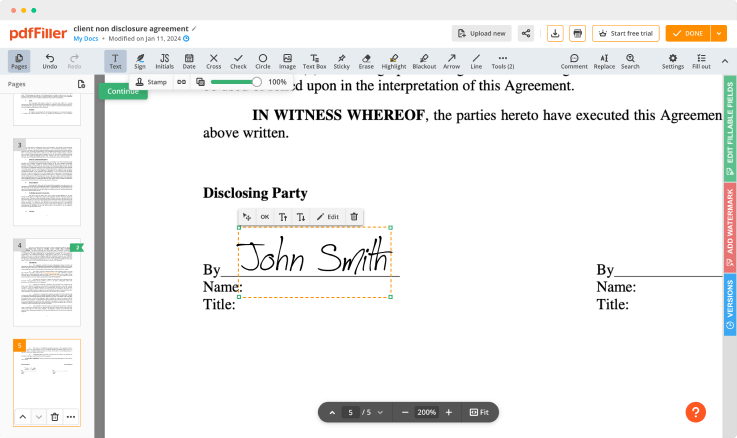
Use powerful editing tools such as typing text, annotating, and highlighting. Add images into your PDF and edit its layout. Change a template’s page order. Add fillable fields and send to sign. Ask other users to fill out the fields and request an attachment if needed. Once a document is completed, download it to your device or save it to cloud.
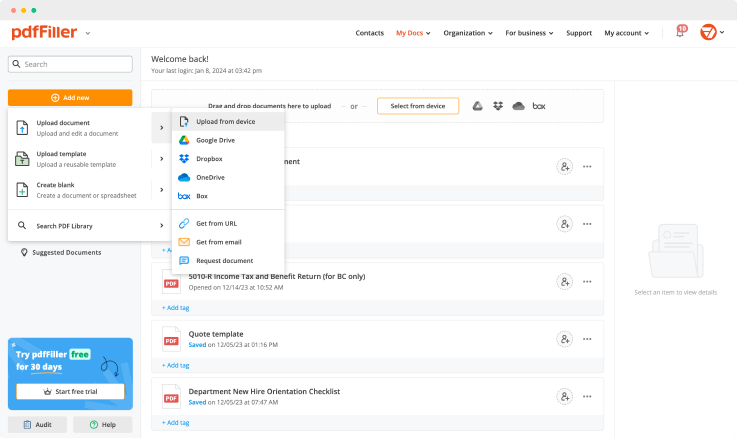
Create a document yourself or upload an existing form using the following methods:
Using pdfFiller, editing documents online has never been as effortless and effective. Boost your workflow and make filling out templates and signing forms a breeze.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Regulate Footnote Record