Regulate Identification Log Gratuito
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

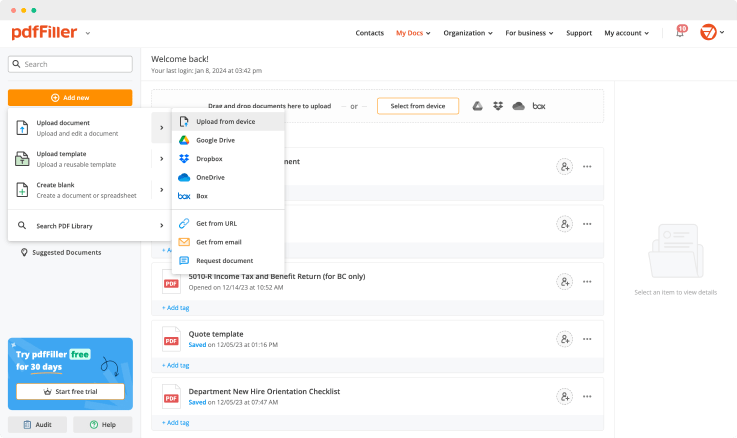
Upload a document

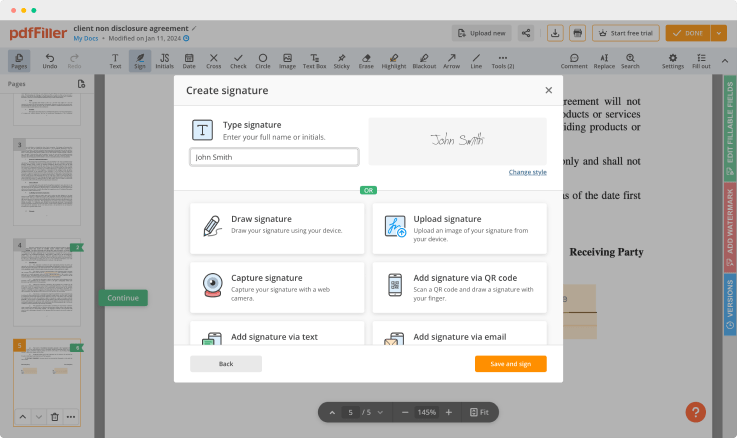
Generate your customized signature

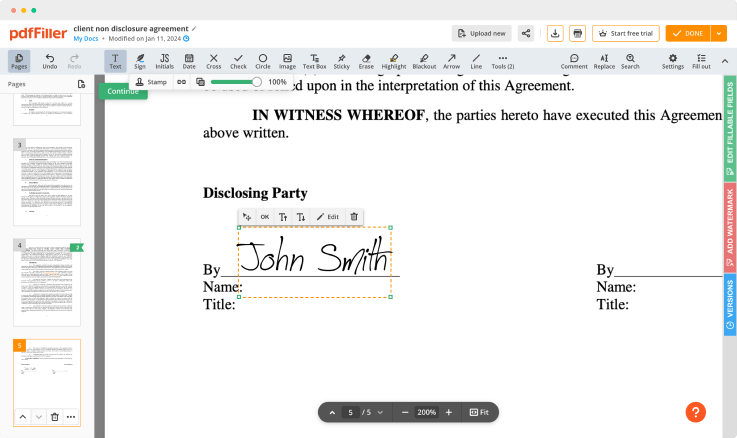
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Identification Log Feature
The Regulate Identification Log feature is designed to streamline the management and tracking of identification documents in your organization. With this tool, you can ensure that all identification records are organized, easily accessible, and compliant with relevant regulations.
Key Features
Centralized storage for all identification documents
Automatic alerts for document expiration
Customizable user access controls
Advanced search and filtering options
Regular audits and reporting capabilities
Potential Use Cases and Benefits
Businesses needing to track employee identification for compliance
Schools managing student identification records efficiently
Healthcare organizations ensuring proper identification for patient records
Government agencies overseeing identification for citizens
This feature resolves your challenges by providing a simple way to keep track of crucial identification records. You can manage your documents effectively, reduce the risk of lost information, and ensure compliance with varying regulations. By utilizing the Regulate Identification Log feature, you gain peace of mind that your organization remains organized and compliant.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What are the regulatory documents?
Regulatory Documents means all dossiers, filings, applications, modifications, amendments, supplements, revisions, reports, submissions, authorizations and approvals, including any IND or NDA, and any reports or amendments necessary to maintain Regulatory Approvals.
What are regulatory documents in clinical trials?
Essential documents are commonly referred to as regulatory documents. ICH GCP guidance defines essential documents as those documents which individually and collectively permit evaluation of the conduct of the clinical trial and the quality of the data produced.
What are essential documents in clinical trials?
ESSENTIAL DOCUMENTS FOR THE CONDUCT OF A CLINICAL TRIAL. Essential Documents are those documents which individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced.
What is in a regulatory binder?
The Regulatory Binder is a template and guidance document for tracking documentation associated with studies involving human subjects. It is designed to help study sites achieve and maintain regulatory compliance and adhere to high standards of practice in the conduct of research involving human subjects.
Does the FDA regulated clinical trials?
The Food and Drug Administration (FDA) regulations for the conduct of clinical trials, which have been in effect since the 1970s, address both GCP and HSP.
How do you write a regulatory document?
Use basic medical writing skills, including correct abbreviation practices, consistent captioning, and table generation. Utilize styles and templates. Describe style guides and their importance. Navigate the communication process necessary for document review and completions. Conduct a literature search.
What is a regulatory document?
Regulatory Documents means the prospectus, annual report, semi-annual report and any other document required under applicable federal securities' law to be delivered by the Fund to Shareholders. Based on 17 documents 17. New List.
What is regulatory writing?
The role of a regulatory writer is to produce regulatory documents (usually taken to refer to documents that are submitted in some form to the health authorities).
Ready to try pdfFiller's? Regulate Identification Log Gratuito
Upload a document and create your digital autograph now.