Secure Quantity Title Gratuito
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

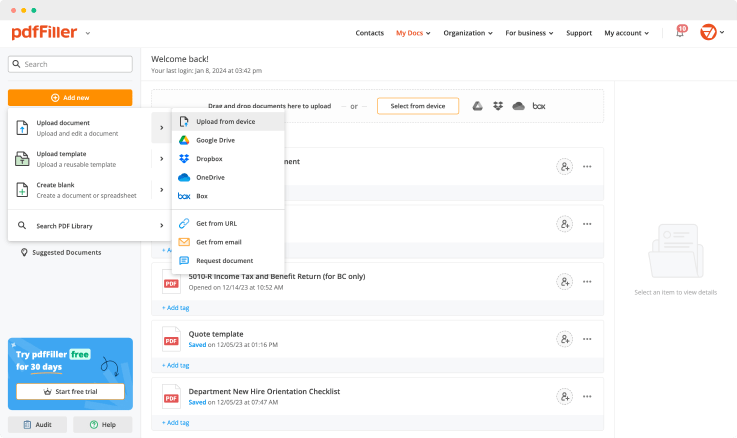
Upload a document

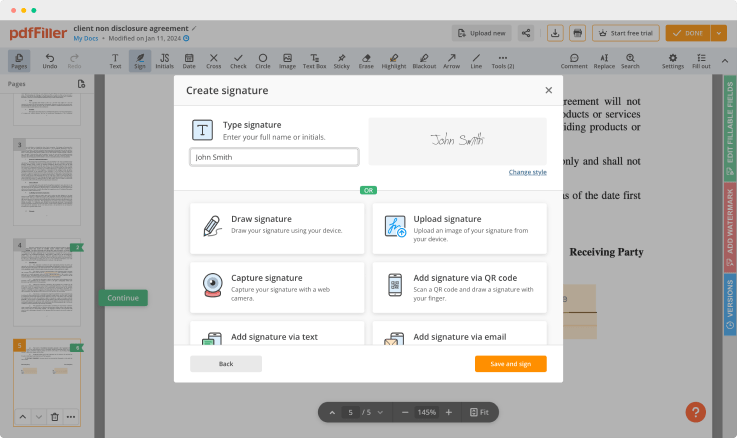
Generate your customized signature

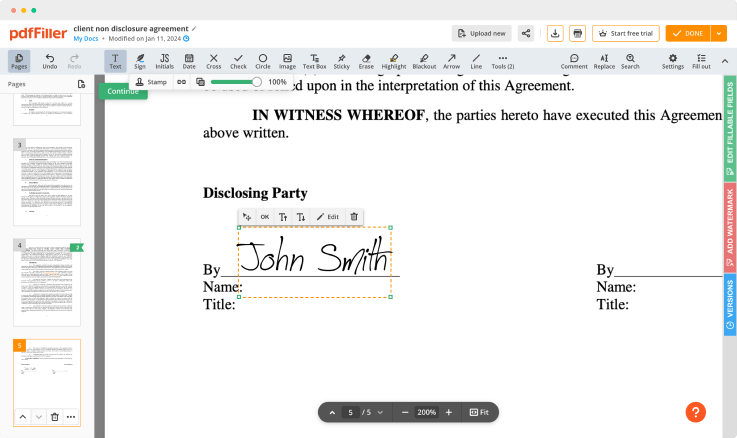
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Secure Quantity Title Feature
The Secure Quantity Title feature provides a reliable way to manage and track your inventory levels with precision. With this feature, you gain clarity over what you own, how much you have, and the ability to make informed decisions about your stock.
Key Features
Real-time inventory tracking
Customizable alerts for low stock levels
User-friendly dashboard for easy navigation
Secure access control to protect sensitive data
Integration with existing inventory management systems
Use Cases and Benefits
Retail businesses can prevent stockouts and overstocking
Manufacturers can maintain optimal production levels
E-commerce platforms can enhance customer satisfaction with accurate availability info
Warehouses can streamline operations with efficient inventory control
This feature addresses common problems like lost sales due to stockouts or wasted resources from excess inventory. By providing you with accurate data and timely alerts, you can make better decisions and improve your overall business efficiency.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How many times can a controlled substance be refilled?
Schedules III and IV controlled substances may be refilled if authorized on the prescription. However, the prescription may only be refilled up to five times within six months after the date of issue. After five refills or after six months, whichever occurs first, a new prescription is required.
How often can control substances be refilled?
According to federal regulations, Schedules III and IV controlled substances may be refilled if authorized on the prescription. These prescriptions may only be refilled up to five times within six months after the date of issue.
How soon can you refill a controlled substance?
According to the federal regulations, controlled medications like Schedules III and IV can only be refilled early on an authorized prescription or usually as early as two days for a 30-day supply. State and local laws may vary slightly as to when you can refill Schedule 3 and 4 drugs.
How long should I wait between Adderall refills?
Do you have to wait 30 days to refill Adderall? Adderall is a Schedule II federally regulated controlled substance. Under federal law, schedule II prescriptions cannot be refilled. For this reason, your health care provider will usually provide multiple prescriptions with a Do not fill until X date written on them.
What are the limitations of refilling a Schedule II controlled substance?
Schedule III and IV controlled substances cannot be filled or refilled more than 5 times or more than 6 months after the date the prescription was issued, whichever occurs first. Schedule II prescriptions cannot be refilled. Under federal law, there is no expiration for a Schedule II prescription.
How many times can a Schedule II drug prescription be refilled Quizlet?
When emergency C-II prescriptions are called in, a prescriber has how many days to furnish the pharmacy with a written and signed prescription hard copy? True or false. C-II prescriptions may be refilled up to 5 times in 6 months. False, C-II prescription refills are legally prohibited.
Can you get a 90-day supply of a controlled substance?
On December 19, 2007, a DEA regulation came into effect that allows a prescriber to issue multiple prescriptions authorizing an individual patient to receive a total of up to a 90-day supply of a Schedule II controlled substance. Every Schedule II prescription must be written on a separate prescription blank.
Can a c2 be written for a 90-day supply?
A. The rule does not stipulate how many prescriptions per schedule II controlled substance may be issued for the 90-day supply. It is up to the practitioner to determine how many prescriptions to be filled sequentially are needed to provide adequate medical care.
Ready to try pdfFiller's? Secure Quantity Title Gratuito
Upload a document and create your digital autograph now.