Signed Consent Gratuito




Join the world’s largest companies
How to Send a PDF for eSignature









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Signed Consent Feature
The Signed Consent feature streamlines the process of obtaining and managing consent legally and efficiently. With this tool, you can secure consent digitally, ensuring that all parties have an easy and clear understanding of agreements.
Key Features
Potential Use Cases and Benefits
This feature addresses the common challenge of managing consent forms manually, which can lead to confusion and mistakes. By using the Signed Consent feature, you reduce errors, save time, and ensure that all necessary approvals are in place. Enjoy peace of mind knowing that you have a reliable system for consent management.
Signed Consent with the swift ease
pdfFiller allows you to Signed Consent quickly. The editor's convenient drag and drop interface ensures fast and user-friendly signing on any operaring system.
Signing PDFs online is a quick and secure method to verify documents anytime and anywhere, even while on the go.
Go through the step-by-step instructions on how to Signed Consent electronically with pdfFiller:
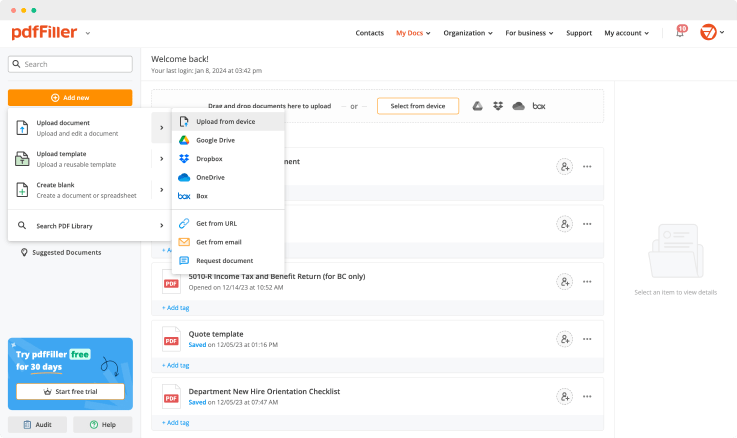
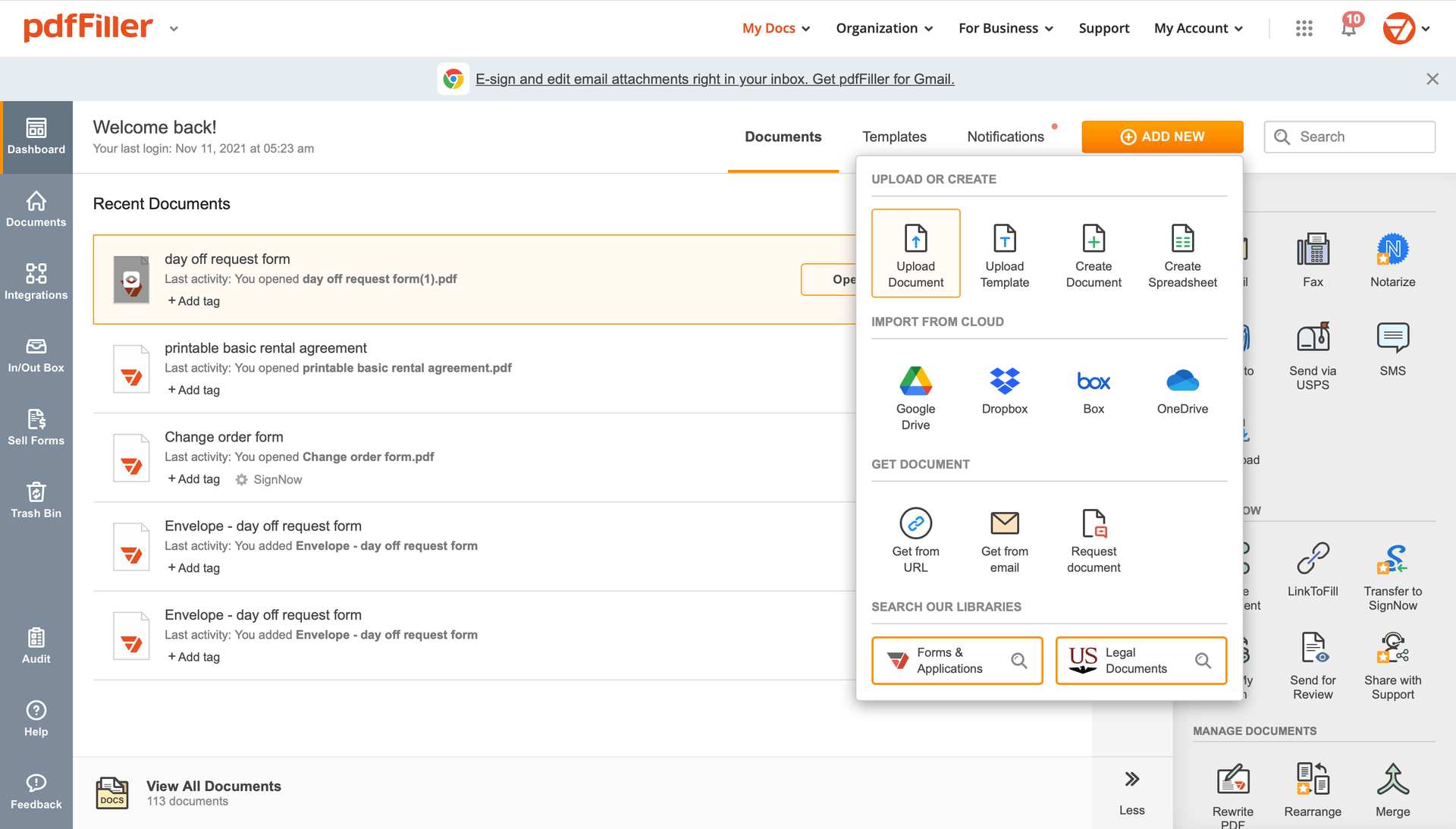
Add the form for eSignature to pdfFiller from your device or cloud storage.
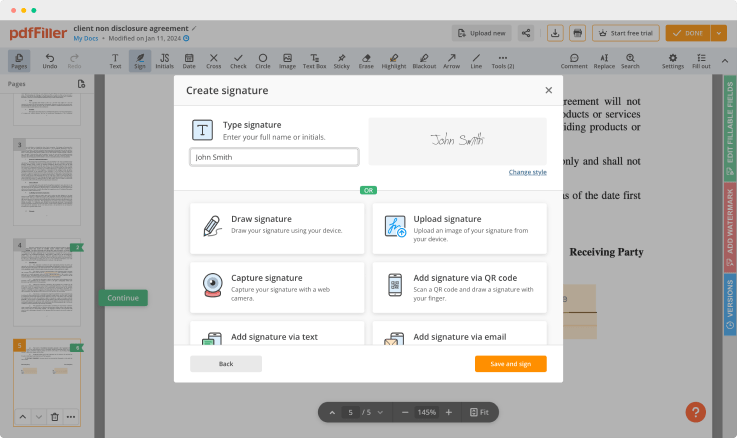
Once the file opens in the editor, hit Sign in the top toolbar.
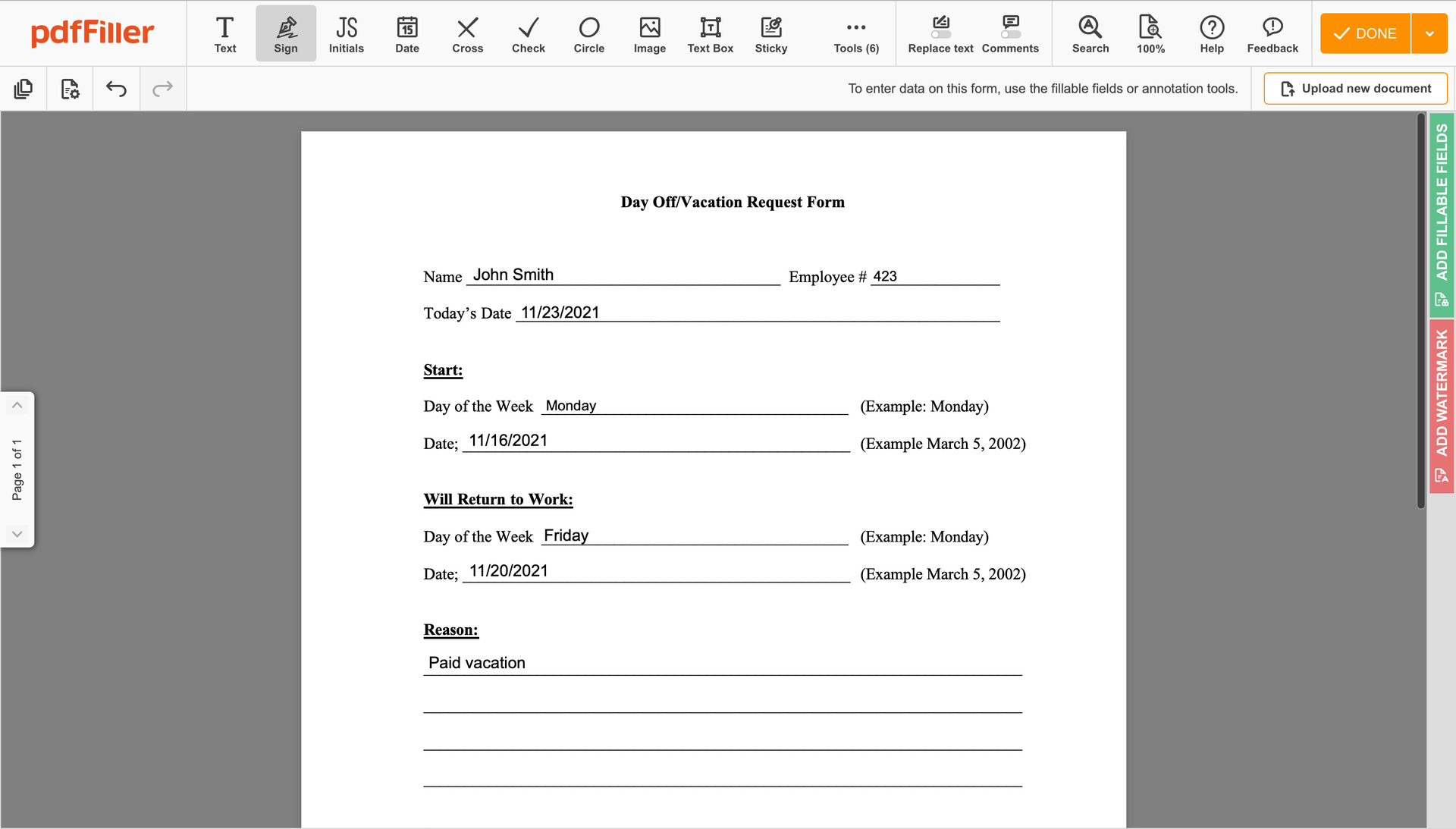
Generate your electronic signature by typing, drawing, or importing your handwritten signature's image from your laptop. Then, click Save and sign.
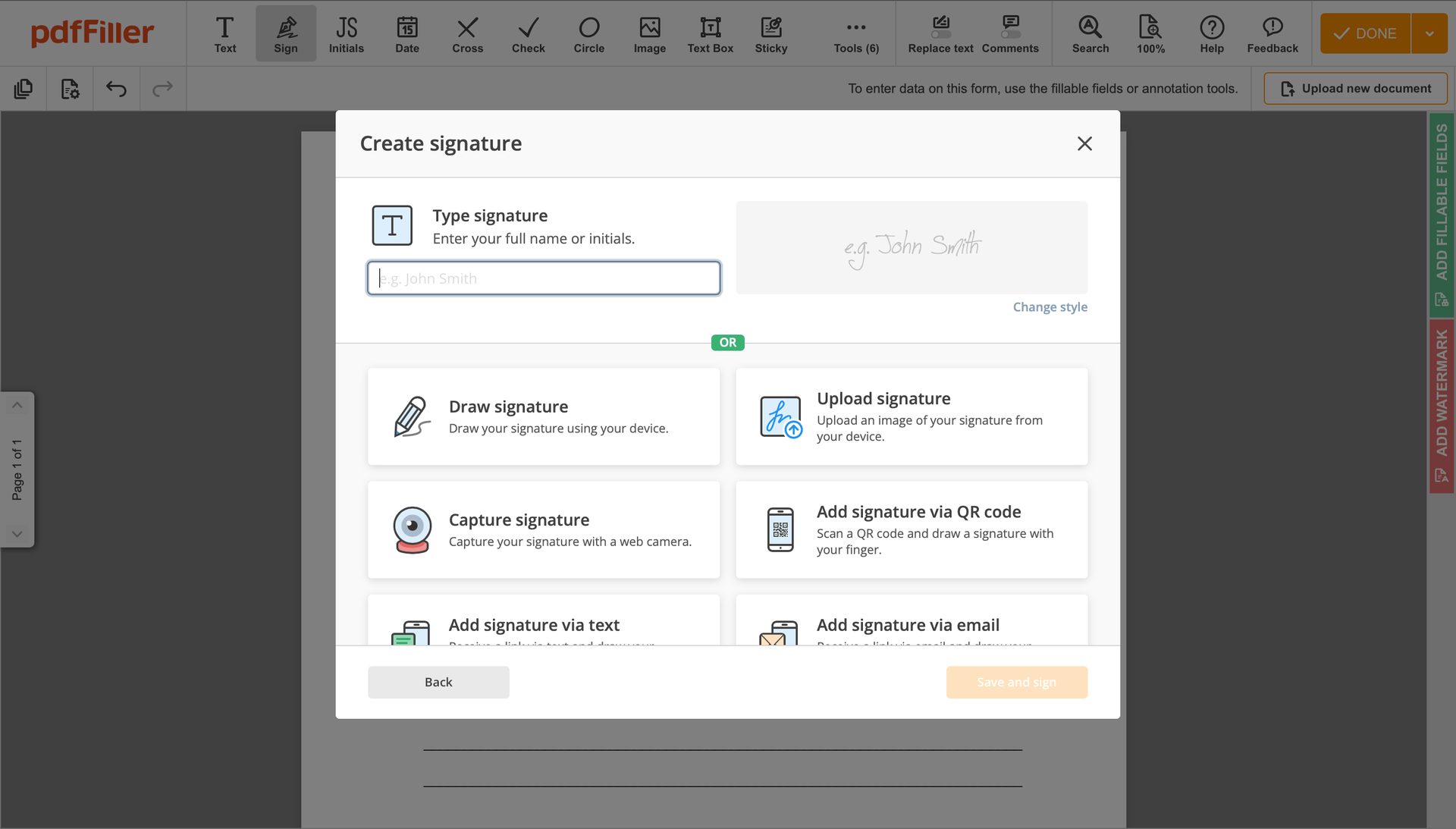
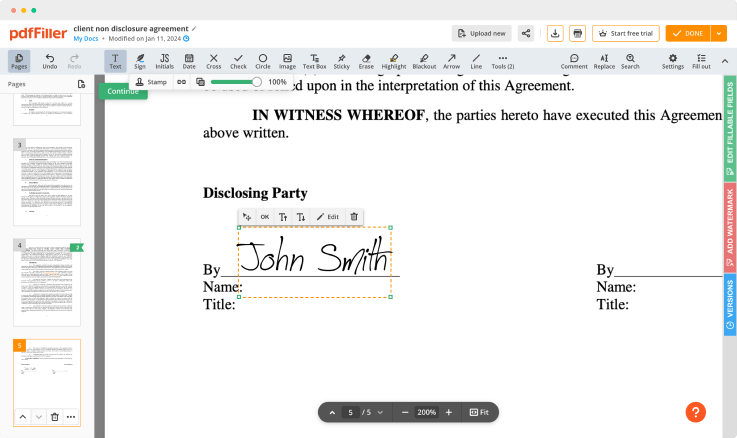
Click anywhere on a form to Signed Consent. You can move it around or resize it utilizing the controls in the floating panel. To use your signature, click OK.
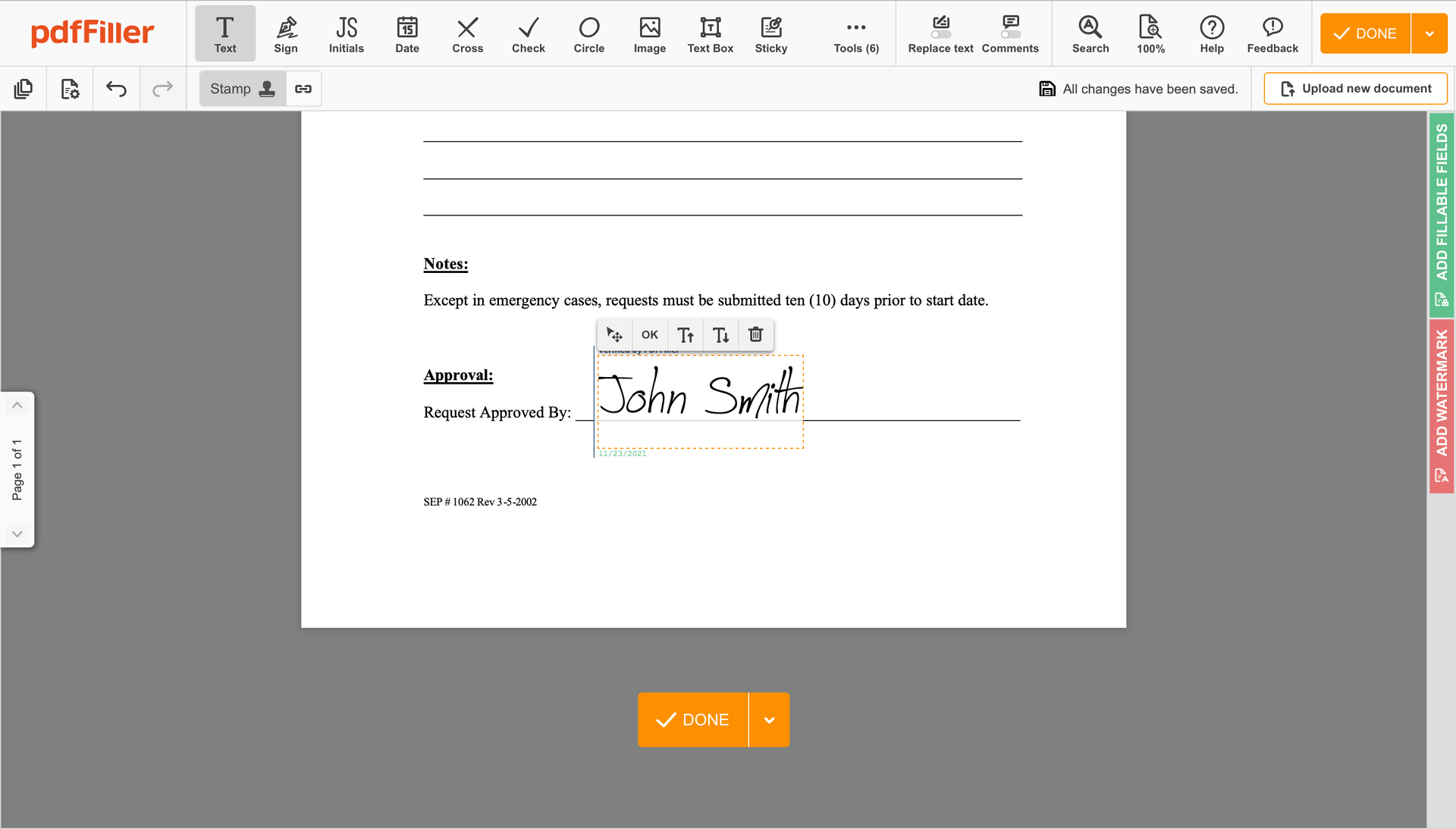
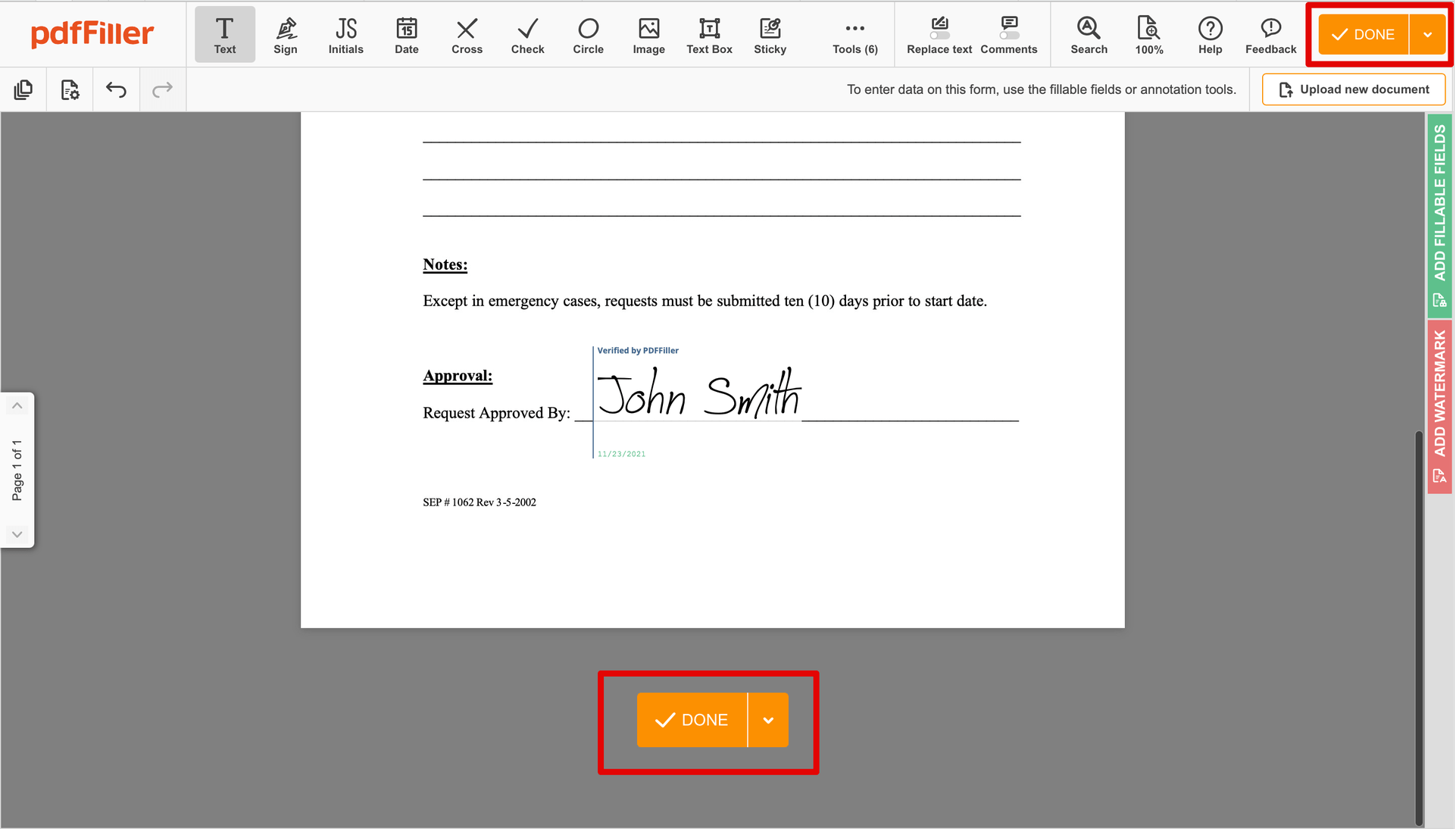
Complete the signing process by clicking DONE below your document or in the top right corner.
After that, you'll go back to the pdfFiller dashboard. From there, you can download a signed copy, print the document, or send it to other people for review or approval.
Are you stuck working with different programs for creating and managing documents? Try this all-in-one solution instead. Use our platform to make the process simple. Create document templates on your own, modify existing forms, integrate cloud services and utilize other useful features within your browser. You can use Signed Consent with ease; all of our features are available to all users. Get the value of full featured platform, for the cost of a lightweight basic app.
How to edit a PDF document using the pdfFiller editor:
For pdfFiller’s FAQs
Ready to try pdfFiller's? Signed Consent Gratuito