Electronegativity Of H - Page 2
What is electronegativity of H?
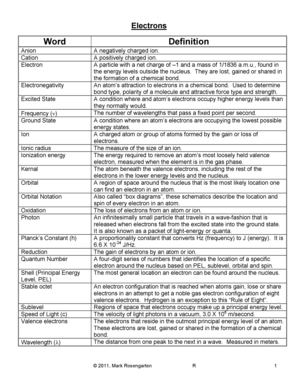
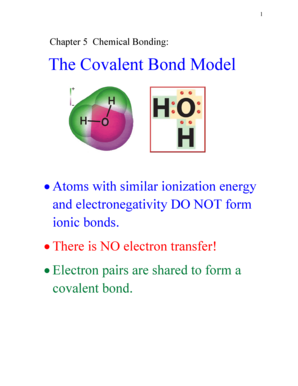
Electronegativity is a property that describes the ability of an atom to attract electrons towards itself in a chemical bond. In the case of hydrogen (H), its electronegativity refers to its tendency to attract electrons when it forms a bond with other atoms.
What are the types of electronegativity of H?
There are no specific types of electronegativity for hydrogen (H) as it is a single element. However, electronegativity values can vary depending on the type of chemical bond hydrogen forms with other elements.
How to complete electronegativity of H
To determine the electronegativity of hydrogen (H) in a molecule or compound, you can follow these steps:
When it comes to creating, editing, and sharing documents online, pdfFiller is the ultimate solution. With unlimited fillable templates and powerful editing tools, users can effortlessly handle their document needs. Say goodbye to the hassle of finding multiple tools for different document tasks. pdfFiller is the only PDF editor you'll ever need to efficiently complete your documents.