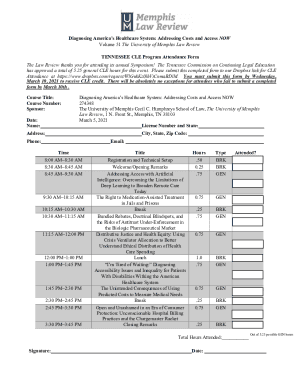
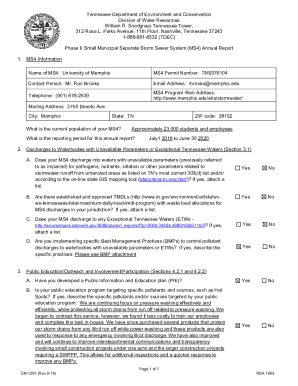
Get the free Summary or Annual Report for Non-Exempt Research - subr
Show details
This document is intended for researchers at Southern University - Baton Rouge to report the status and details of their approved research projects to the Institutional Review Board (IRB), including
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign summary or annual report

Edit your summary or annual report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your summary or annual report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing summary or annual report online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Check your account. It's time to start your free trial.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit summary or annual report. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
The use of pdfFiller makes dealing with documents straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out summary or annual report

How to fill out Summary or Annual Report for Non-Exempt Research
01
Gather all relevant research data and findings from the reporting period.
02
Ensure that all team members have contributed their sections or findings for accuracy.
03
Format the report according to the guidelines provided by the funding agency or organization.
04
Write a clear summary of the research objectives and outcomes in the introduction.
05
Include sections for methodology, results, and discussions in a structured manner.
06
Use tables, figures, and charts to visually present data where appropriate.
07
Check for compliance with any ethical or regulatory requirements in your section.
08
Review the entire report for grammar, clarity, and consistency.
09
Submit the report by the deadline, ensuring you have followed all submission instructions.
Who needs Summary or Annual Report for Non-Exempt Research?
01
Researchers conducting non-exempt studies funded by government or private agencies.
02
Institutions that oversee research compliance and funding administration.
03
Ethics review boards that require oversight of research activities.
04
Funding agencies that need reports to assess the impact and compliance of their investments.
Fill
form
: Try Risk Free






People Also Ask about
What is an example of an exempt IRB?
Some examples of Exempt research are: surveys or interviews. benign behavioral interventions. passive observation of public behavior without collection of identifiers.
What is the difference between exempt research and non exempt research?
Although studies that qualify for exempt status do not have the same federal requirements for research involving human subjects as non-exempt studies, investigators still have a responsibility to protect the rights and welfare of their subjects, adhere to UCB policies, and conduct their research in ance with the
Which type of IRB review does not require approval?
“Exempt” human subjects research is a sub-set of research involving human subjects that does not require comprehensive IRB review and approval because the only research activity involving the human subjects falls into one or more specific exemption categories as defined by the Common Rule.
What projects are IRB exempt?
Exempt Categories: Education research. Surveys, interviews, educational tests, public observations (that do not involve children) Benign behavioral interventions. Analysis of previously-collected, identifiable info/specimens. Federal research/demonstration projects. Taste and food evaluation studies.
What is an example of an exempt IRB review?
Examples of Commonly Exempted Research Research on educational practices or educational curriculums. Benign behavioral interventions with adult subjects. Anonymous surveys or interviews on non-sensitive topics. Passive observation of public behavior without collection of identifiers.
When can a study be considered exempt from IRB review?
Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal IRB regulations. Studies that qualify for exemption must be submitted to the IRB for review before starting the research.
Does all research need to for through the IRB review if not which ones are exempt?
Yes. If you are gathering data from people and you plan to publish your findings, you absolutely need to go through IRB. Even for things that don't strictly need IRB approval, journals are still going to want to see that an IRB officially declared your research to be ``exempt.''
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Summary or Annual Report for Non-Exempt Research?
The Summary or Annual Report for Non-Exempt Research is a document that outlines the activities and findings of research projects that do not qualify for exemptions under federal regulations. This report ensures compliance with regulatory standards and provides transparency regarding the research conducted.
Who is required to file Summary or Annual Report for Non-Exempt Research?
Researchers and institutions that conduct non-exempt research involving human subjects are required to file the Summary or Annual Report. This includes academic institutions, private organizations, and any entity receiving federal funding for research.
How to fill out Summary or Annual Report for Non-Exempt Research?
To fill out the Summary or Annual Report, researchers should gather relevant data on their research activities, including participant demographics, methodologies used, outcomes, and any adverse events. They will need to complete the designated form provided by the overseeing regulatory body or institution, ensuring all sections are accurately filled out and supported by documentation.
What is the purpose of Summary or Annual Report for Non-Exempt Research?
The purpose of the Summary or Annual Report is to provide oversight and accountability for research involving human subjects, ensure compliance with ethical standards, and facilitate communication between researchers and regulatory authorities regarding research practices and outcomes.
What information must be reported on Summary or Annual Report for Non-Exempt Research?
The report must include information such as the title of the research project, the names of the principal investigators, the objectives of the research, the number of participants, a summary of findings, any ethical concerns or issues encountered, and plans for future research. Additionally, any adverse events or unanticipated problems must also be reported.
Fill out your summary or annual report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Summary Or Annual Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.