Get the free S&C-10-12-CLIA - cms
Show details
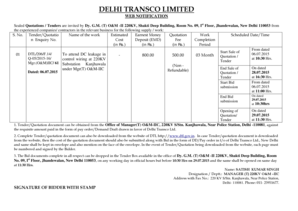
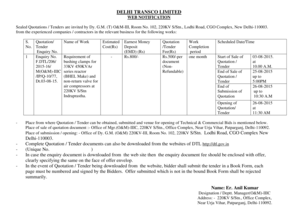
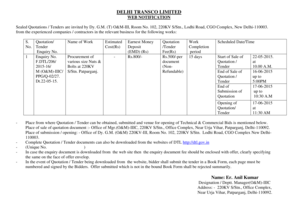
This memorandum discusses the revised survey procedures and interpretive guidelines under CLIA to facilitate electronic exchange of laboratory information and enhance health information technology
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign sc-10-12-clia - cms
Edit your sc-10-12-clia - cms form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your sc-10-12-clia - cms form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit sc-10-12-clia - cms online
To use the services of a skilled PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit sc-10-12-clia - cms. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out sc-10-12-clia - cms
How to fill out S&C-10-12-CLIA
01
Obtain the S&C-10-12-CLIA form from the relevant regulatory agency's website.
02
Read the instructions carefully to understand the requirements for completion.
03
Fill in the facility information, including name, address, and contact details.
04
Provide the type of laboratory services offered by the facility.
05
Indicate the ownership type of the laboratory (e.g., individual, partnership, corporation).
06
Specify the laboratory director's name and qualifications.
07
Complete sections regarding the laboratory's compliance with CLIA regulations.
08
Include any necessary supporting documents, such as proof of qualifications.
09
Review the filled-out form to ensure all information is accurate and complete.
10
Submit the form according to the guidelines provided in the instructions.
Who needs S&C-10-12-CLIA?
01
Laboratories that perform clinical tests and require CLIA certification.
02
Healthcare facilities offering laboratory services needing to demonstrate compliance.
03
Entities seeking federal funding or reimbursements for laboratory services.
Fill
form
: Try Risk Free






People Also Ask about
What are three examples of CLIA waived tests?
Clinical Laboratory Improvement Amendments (CLIA) - developed to improve laboratory testing. waived tests. moderate-complexity tests. high-complexity tests. quality control measures. calibration. control samples. reagents.
What is a CLIA license?
Common CLIA-Waived Test Examples: Urinalysis (dipstick) Rapid strep and flu tests. Pregnancy tests ()
What is a clinical laboratory improvement amendment CLIA waived test?
CLIA-waived tests are diagnostic analyses that have been designated by the Centers for Medicare & Medicaid Services (CMS) as “waived” under the Clinical Laboratory Improvement Amendments (CLIA) regulations. These tests are considered simple to perform and carry a low risk of incorrect results when conducted properly.
What does CLIA mean in medical billing?
Overview. This process involves compliance with the federal Clinical Laboratory Improvement Amendments (CLIA) of 1988, which requires that all laboratories testing human specimens be certified by the federal government. The Los Angeles LFS Office manages the federal CLIA program in California.
What are three types of CLIA licenses?
What is a CLIA Categorization? The FDA categorizes clinical laboratory tests by their complexity — from the least to the most complex: waived tests, moderate complexity tests, and high complexity tests.
What does the name CLIA mean?
The name Clia is primarily a female name of Greek origin that means To Praise, Acclaim. Form of the name Cleopatra.
What are the 3 CLIA categories?
What is a CLIA Categorization? The FDA categorizes clinical laboratory tests by their complexity — from the least to the most complex: waived tests, moderate complexity tests, and high complexity tests.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is S&C-10-12-CLIA?
S&C-10-12-CLIA is a form used by laboratories to report compliance with the Clinical Laboratory Improvement Amendments (CLIA) regulations. It helps ensure that laboratories meet the quality standards set for laboratory testing.
Who is required to file S&C-10-12-CLIA?
Laboratories that perform testing on human specimens and are subject to CLIA regulations are required to file S&C-10-12-CLIA. This includes facilities that conduct clinical laboratory tests for diagnosis, prevention, or treatment of diseases.
How to fill out S&C-10-12-CLIA?
To fill out S&C-10-12-CLIA, laboratories should provide accurate information regarding their compliance with CLIA regulations, including testing methods, certifications, and personnel qualifications. Detailed instructions are typically provided with the form.
What is the purpose of S&C-10-12-CLIA?
The purpose of S&C-10-12-CLIA is to collect and verify information from laboratories to ensure they adhere to CLIA standards, maintain quality laboratory practices, and improve patient safety and care.
What information must be reported on S&C-10-12-CLIA?
Laboratories must report information including their CLIA certificate number, the types of tests performed, compliance with quality standards, lab personnel qualifications, and any relevant changes in testing methodology or personnel.
Fill out your sc-10-12-clia - cms online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Sc-10-12-Clia - Cms is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.