Get the free INVESTIGATIONAL NEW DRUG IND DRUG INFORMATION SHEET A - academicdepartments musc
Show details
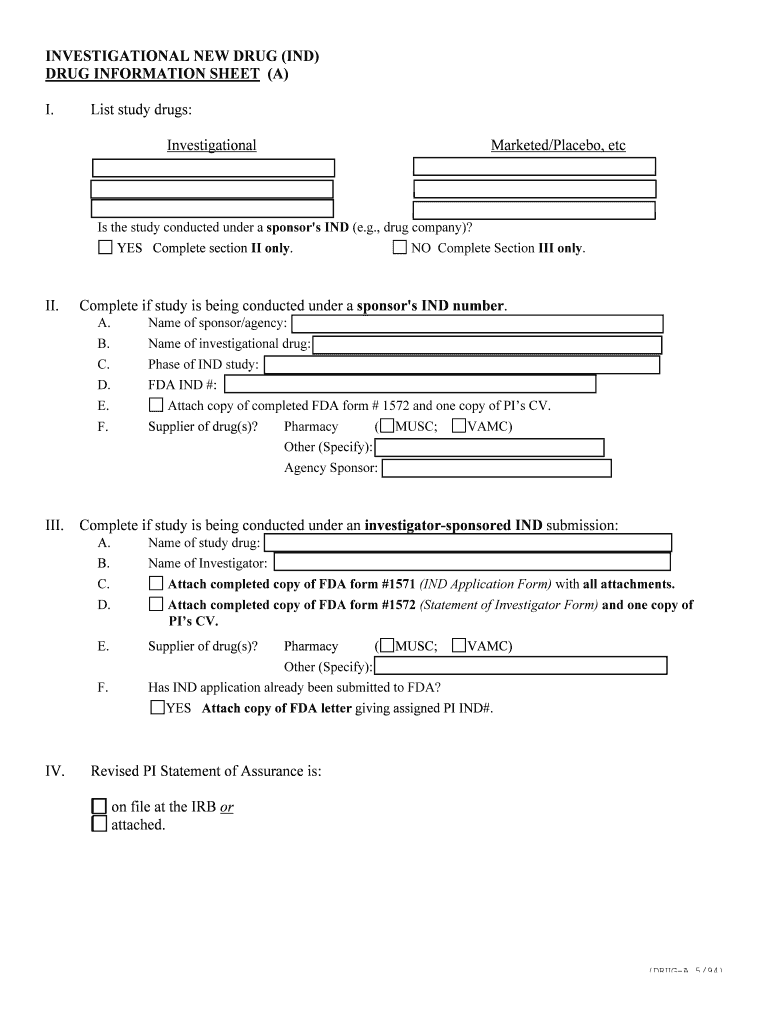
INVESTIGATIONAL NEW DRUG (IND) DRUG INFORMATION SHEET (A) I. List study drugs: Investigational Marketed/Placebo, etc Is the study conducted under a sponsor's IND (e.g., drug company)? YES Complete
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign investigational new drug ind

Edit your investigational new drug ind form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your investigational new drug ind form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit investigational new drug ind online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit investigational new drug ind. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out investigational new drug ind

How to Fill Out Investigational New Drug (IND)?
01
Obtain the required forms: Begin by obtaining the necessary forms for filing an Investigational New Drug (IND) application. These forms can typically be obtained from the regulatory authorities such as the U.S. Food and Drug Administration (FDA).
02
Provide basic information: Fill out the provided sections that require basic information about the drug, such as its proposed name, chemical structure, and dosage form. Include details about the manufacturer, sponsor, and any previous or ongoing clinical studies.
03
Define the drug's intended use: Clearly describe the drug's intended use, including the conditions or indications it aims to treat or prevent. Explain the rationale behind the development of the drug and provide scientific and clinical justification.
04
Present preclinical data: Include preclinical data that supports the safety and efficacy of the drug. These can include results from in vitro and in vivo studies, animal studies, and any relevant pharmacological or toxicological data. It is crucial to demonstrate the potential benefits of the investigational drug and assess any potential risks.
05
Submit study protocols: Include detailed study protocols that outline the design and objectives of any clinical trials or investigations planned to evaluate the drug. These protocols should describe the population to be studied, the endpoints, and the proposed methodology for data collection and analysis.
06
Provide information on manufacturing: Include information about the drug's manufacturing process, quality control procedures, and specifications. Provide details on the facilities where the drug will be manufactured, including any relevant certifications or compliance with Good Manufacturing Practices (GMP).
07
Outline the drug's labeling: Describe the proposed labeling and packaging of the drug, including information on dosage, administration instructions, and any necessary warnings or precautions. This section should comply with the applicable regulations and guidelines.
08
Include any additional information: If applicable, provide any additional information requested by the regulatory authorities. This may include specific safety data, drug interactions, or information on special populations.
Who Needs Investigational New Drug (IND)?
01
Pharmaceuticals and biotechnology companies: Companies involved in the development of new drugs or biologics for human use need to file an IND application to seek approval for conducting clinical trials. This includes both large pharmaceutical companies and smaller biotech startups.
02
Researchers and academicians: Scientists conducting clinical studies to assess the safety and efficacy of investigational drugs or therapies also need to obtain an IND. This applies to academic institutions, hospitals, and research organizations.
03
Contract research organizations (CROs): CROs that provide a wide range of research services, including clinical trial management, play a crucial role in helping sponsors fill out and submit IND applications on behalf of their clients.
Note: The specific requirements and procedures for filling out an IND may vary by country and regulatory authority. It is important to consult the relevant guidelines and seek professional advice to ensure compliance with the applicable regulations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is investigational new drug ind?
An investigational new drug (IND) is a drug that is being investigated or studied for its safety and effectiveness in humans.
Who is required to file investigational new drug ind?
Sponsors or researchers conducting clinical trials on new drugs are required to file an investigational new drug (IND) application with the FDA.
How to fill out investigational new drug ind?
To fill out an investigational new drug (IND) application, sponsors or researchers must provide detailed information about the drug, its proposed use, study protocols, and safety data.
What is the purpose of investigational new drug ind?
The purpose of an investigational new drug (IND) application is to allow researchers to study the safety and effectiveness of a new drug in humans before it can be approved for marketing.
What information must be reported on investigational new drug ind?
Information that must be reported on an investigational new drug (IND) application includes the chemical composition of the drug, its pharmacological properties, data from animal studies, and proposed clinical study plans.
How do I make changes in investigational new drug ind?
The editing procedure is simple with pdfFiller. Open your investigational new drug ind in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I edit investigational new drug ind on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign investigational new drug ind. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
How do I complete investigational new drug ind on an Android device?
Use the pdfFiller mobile app to complete your investigational new drug ind on an Android device. The application makes it possible to perform all needed document management manipulations, like adding, editing, and removing text, signing, annotating, and more. All you need is your smartphone and an internet connection.
Fill out your investigational new drug ind online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Investigational New Drug Ind is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.