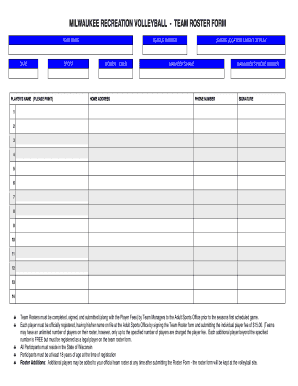
Get the free TITULAR LABORATORIOS BIOLOGICOS FARMACEUTICOS LTDA LABIFAR S - web sivicos gov
Show details
Institute Nacional de Vigilancia de Medicamentos y Aliments INDIA Minister de la Protection Social Replica de Colombia EDICT No.2009001626 Boot D.C. 17 de Junior de 2009 EXPEDIENT: 19952063 RADIATION:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign titular laboratorios biologicos farmaceuticos

Edit your titular laboratorios biologicos farmaceuticos form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your titular laboratorios biologicos farmaceuticos form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing titular laboratorios biologicos farmaceuticos online
To use the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit titular laboratorios biologicos farmaceuticos. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out titular laboratorios biologicos farmaceuticos

How to fill out titular laboratorios biologicos farmaceuticos:
01
Start by gathering all the necessary information and documents required for the application process. This may include personal identification, qualifications, certifications, and any relevant experience in the pharmaceutical industry.
02
Ensure that you understand the requirements and regulations for obtaining titular laboratorios biologicos farmaceuticos. Familiarize yourself with the specific guidelines and criteria set forth by the relevant regulatory bodies.
03
Carefully fill out the application form, providing accurate and complete information. Double-check all the details to avoid any errors or omissions that may delay the process.
04
Attach all the requested supporting documents to your application. These may include copies of your academic degrees, professional licenses, and any other relevant certifications.
05
Submit your completed application along with the necessary documents to the appropriate regulatory authority. Follow their specified submission procedures, whether it be through an online portal or by mail.
06
Pay the required fees associated with the application. Ensure that you have the correct payment method and that the fees are submitted in a timely manner. Keep a copy of the payment receipt for your records.
07
Track the progress of your application and be prepared to respond to any additional requests for information or documentation. Stay in communication with the regulatory authority to ensure a smooth and efficient process.
Who needs titular laboratorios biologicos farmaceuticos?
01
Pharmaceutical companies: Titular laboratorios biologicos farmaceuticos are essential for pharmaceutical companies involved in the research, development, and manufacturing of biological medicines. This certification ensures compliance with the necessary regulatory standards.
02
Medical research institutions: Institutions engaged in medical research, particularly in the field of biotechnology and biological medicines, may require titular laboratorios biologicos farmaceuticos to ensure the quality and safety of their research activities.
03
Regulatory authorities: Government regulatory authorities responsible for overseeing the pharmaceutical industry often require pharmaceutical companies to hold titular laboratorios biologicos farmaceuticos as a condition for market authorization and compliance with regulatory standards.
In conclusion, filling out titular laboratorios biologicos farmaceuticos requires careful attention to detail, adherence to regulatory guidelines, and the submission of necessary documents. This certification is crucial for pharmaceutical companies, medical research institutions, and regulatory authorities involved in the development and monitoring of biological medicines.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is titular laboratorios biologicos farmaceuticos?
Titular laboratorios biologicos farmaceuticos refers to pharmaceutical companies that are in charge of the manufacture and distribution of biological and pharmaceutical products.
Who is required to file titular laboratorios biologicos farmaceuticos?
Pharmaceutical companies that produce biological and pharmaceutical products are required to file titular laboratorios biologicos farmaceuticos.
How to fill out titular laboratorios biologicos farmaceuticos?
Titular laboratorios biologicos farmaceuticos must be filled out by providing detailed information about the biological and pharmaceutical products being manufactured and distributed by the company.
What is the purpose of titular laboratorios biologicos farmaceuticos?
The purpose of titular laboratorios biologicos farmaceuticos is to ensure transparency and compliance with regulations in the pharmaceutical industry.
What information must be reported on titular laboratorios biologicos farmaceuticos?
Information such as the name of the products, their composition, manufacturing processes, and distribution channels must be reported on titular laboratorios biologicos farmaceuticos.
Can I create an eSignature for the titular laboratorios biologicos farmaceuticos in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your titular laboratorios biologicos farmaceuticos and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
Can I edit titular laboratorios biologicos farmaceuticos on an Android device?
You can make any changes to PDF files, such as titular laboratorios biologicos farmaceuticos, with the help of the pdfFiller mobile app for Android. Edit, sign, and send documents right from your mobile device. Install the app and streamline your document management wherever you are.
How do I complete titular laboratorios biologicos farmaceuticos on an Android device?
Use the pdfFiller Android app to finish your titular laboratorios biologicos farmaceuticos and other documents on your Android phone. The app has all the features you need to manage your documents, like editing content, eSigning, annotating, sharing files, and more. At any time, as long as there is an internet connection.
Fill out your titular laboratorios biologicos farmaceuticos online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Titular Laboratorios Biologicos Farmaceuticos is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.