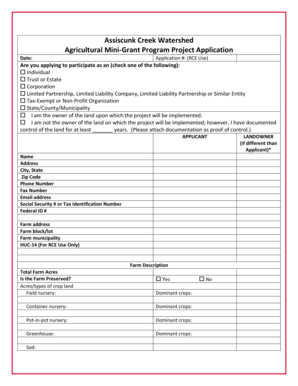
Get the free Controlled Substances SOP REV 5 for ASCLD LABdoc OVB Annual Report 2006 - cacj
Show details
Case 3:05cr00167WHA Document 6372 Filed 08/07/2006-Page 1 of 67 SFPD Crime Lab Controlled Substances SOP Version 06/23/05 REV.5 Approved: MB San Francisco Police Department Criminalistics Laboratory
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign controlled substances sop rev

Edit your controlled substances sop rev form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your controlled substances sop rev form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing controlled substances sop rev online
To use the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit controlled substances sop rev. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out controlled substances sop rev

How to fill out controlled substances SOP rev:
01
Begin by reviewing the existing controlled substances SOP (Standard Operating Procedure). Familiarize yourself with the current guidelines and procedures in place.
02
Make note of any necessary updates or revisions to the SOP. This could include changes in regulations, procedures, or documentation requirements.
03
Gather all relevant information and documentation needed to complete the revision process. This may include data on controlled substances inventory, storage, ordering, disposal, and record-keeping.
04
Consult with relevant stakeholders, such as pharmacists, compliance officers, and administrators, to ensure accurate and comprehensive information is included in the revised SOP.
05
Evaluate and revise each section of the SOP systematically. Provide clear and concise instructions and guidelines for each step of the controlled substances management process.
06
Incorporate any new policies or procedures that have been implemented since the last SOP revision. Ensure that these changes are reflected accurately.
07
Document any changes made to the SOP in a revision log, including the date, signature, and initials of the person making the revisions.
08
Once the revision process is complete, review the revised SOP with key stakeholders to ensure their understanding and compliance.
09
Distribute the revised SOP to all relevant personnel and provide appropriate training or education to ensure adherence to the updated guidelines.
10
Regularly monitor and evaluate the effectiveness of the revised SOP, making any necessary adjustments as needed.
Who needs controlled substances SOP rev:
01
Organizations or facilities that handle or dispense controlled substances.
02
Healthcare providers, including physicians, nurses, pharmacists, and other medical professionals who administer or prescribe controlled substances.
03
Compliance officers responsible for ensuring adherence to regulations and guidelines related to controlled substances management.
04
Administrators or managers of healthcare facilities or organizations, who have overall responsibility for maintaining accurate and up-to-date SOPs.
05
Regulatory agencies or bodies that oversee and enforce controlled substances regulations and policies.
06
Auditors or inspectors who may review and assess the organization's compliance with controlled substances regulations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute controlled substances sop rev online?
pdfFiller has made it easy to fill out and sign controlled substances sop rev. You can use the solution to change and move PDF content, add fields that can be filled in, and sign the document electronically. Start a free trial of pdfFiller, the best tool for editing and filling in documents.
Can I edit controlled substances sop rev on an Android device?
With the pdfFiller Android app, you can edit, sign, and share controlled substances sop rev on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
How do I complete controlled substances sop rev on an Android device?
Use the pdfFiller mobile app and complete your controlled substances sop rev and other documents on your Android device. The app provides you with all essential document management features, such as editing content, eSigning, annotating, sharing files, etc. You will have access to your documents at any time, as long as there is an internet connection.
What is controlled substances sop rev?
Controlled substances sop rev refers to the Standard Operating Procedure for the handling and management of controlled substances.
Who is required to file controlled substances sop rev?
Any organization or individual who handles controlled substances is required to file controlled substances sop rev.
How to fill out controlled substances sop rev?
To fill out controlled substances sop rev, one must follow the guidelines and procedures outlined in the Standard Operating Procedure document.
What is the purpose of controlled substances sop rev?
The purpose of controlled substances sop rev is to ensure the safe and proper handling, storage, and disposal of controlled substances to prevent misuse or diversion.
What information must be reported on controlled substances sop rev?
The information required to be reported on controlled substances sop rev includes details on the acquisition, use, storage, and disposal of controlled substances.
Fill out your controlled substances sop rev online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Controlled Substances Sop Rev is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.