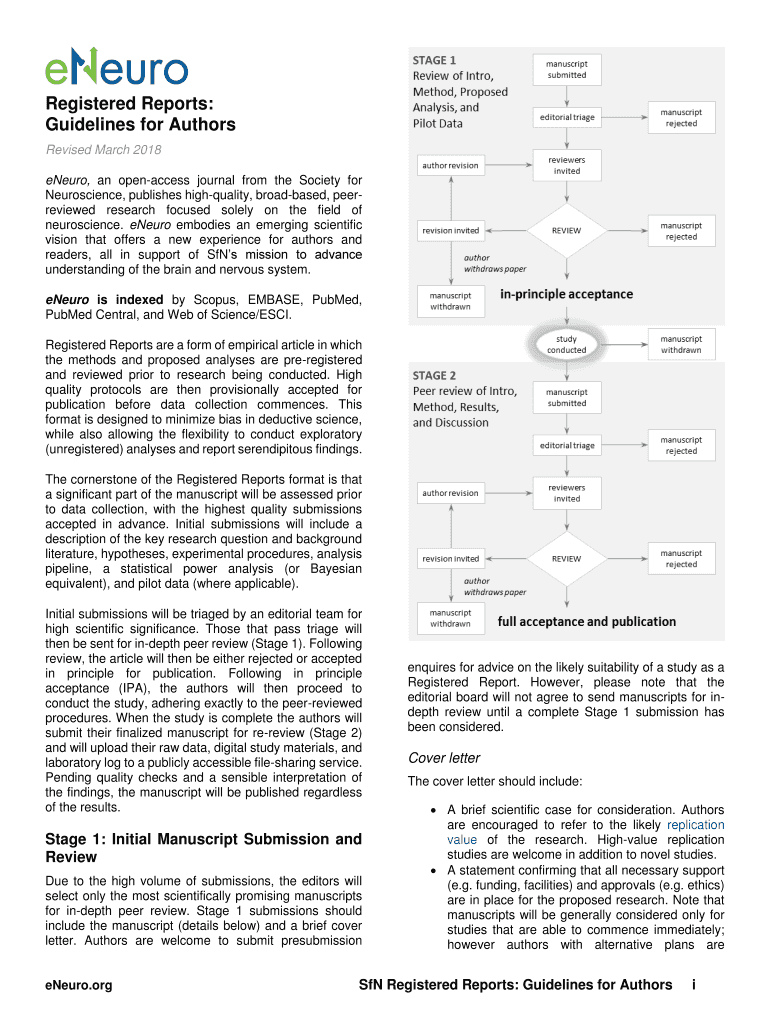
Get the free Registered Reports: Guidelines for Authors - eNeuro
Show details
Registered Reports:
Guidelines for Authors
Revised March 2018
Enduro, an open access journal from the Society for
Neuroscience, publishes high quality, broad based, peer reviewed research focused
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign registered reports guidelines for

Edit your registered reports guidelines for form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your registered reports guidelines for form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit registered reports guidelines for online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit registered reports guidelines for. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out registered reports guidelines for

How to fill out registered reports guidelines for
01
Start by thoroughly reading the registered reports guidelines provided by the respective organization or journal.
02
Familiarize yourself with the format, structure, and content requirements specified in the guidelines.
03
Begin by preparing the title and abstract, ensuring they succinctly describe the study and its objectives.
04
Craft a detailed introduction section that clearly outlines the scientific background, objectives, and rationale behind the study.
05
Clearly define the research question(s) and hypothesis(es), providing a strong justification for their importance.
06
Describe the experimental design, methodology, and data analysis plan in a comprehensive and transparent manner.
07
Include any necessary pilot data, sample size calculations, and statistical considerations.
08
Provide a pre-registration or pre-analysis plan, specifying the primary outcomes, variables, and analyses to be conducted.
09
Discuss potential challenges, limitations, and alternative approaches, demonstrating a thoughtful approach to potential pitfalls.
10
Explain the anticipated ethical considerations, including participant recruitment, informed consent, and data handling.
11
Clearly outline the planned statistical analyses and any exploratory or secondary analyses that may be conducted.
12
Include a detailed reference section to support your scientific claims and provide context to your study.
13
Review and proofread the filled-out registered reports guidelines to ensure clarity, coherence, and accuracy.
14
Submit the completed registered reports guidelines according to the submission instructions provided by the organization or journal.
15
Await feedback and revisions, being prepared to address any reviewer comments or concerns.
16
Once approved, proceed with conducting the study and adhere to the registered reports guidelines throughout the research process.
Who needs registered reports guidelines for?
01
Registered reports guidelines are primarily needed by researchers who want to enhance the credibility and reproducibility of their research.
02
Academics, scientists, and professionals across various fields who wish to pre-register their study protocols and eliminate biases may benefit from these guidelines.
03
Journals or organizations interested in promoting transparent and robust research practices may use registered reports guidelines for authors submitting their studies.
04
The guidelines can also be useful for research ethics committees or institutional review boards (IRBs) that evaluate study protocols for ethical and methodological rigor.
05
Those seeking to improve the quality and reliability of scientific research as a whole can utilize registered reports guidelines as a valuable resource.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete registered reports guidelines for online?
Easy online registered reports guidelines for completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
Can I create an electronic signature for the registered reports guidelines for in Chrome?
Yes, you can. With pdfFiller, you not only get a feature-rich PDF editor and fillable form builder but a powerful e-signature solution that you can add directly to your Chrome browser. Using our extension, you can create your legally-binding eSignature by typing, drawing, or capturing a photo of your signature using your webcam. Choose whichever method you prefer and eSign your registered reports guidelines for in minutes.
How do I edit registered reports guidelines for straight from my smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing registered reports guidelines for, you need to install and log in to the app.
What is registered reports guidelines for?
Registered reports guidelines are designed to improve the rigor and reproducibility of scientific research by outlining a process for pre-registering research plans before data collection.
Who is required to file registered reports guidelines for?
Researchers, scientists, and academics who are conducting studies and experiments that require a high level of transparency and quality control are required to file registered reports guidelines.
How to fill out registered reports guidelines for?
To fill out registered reports guidelines, researchers must provide detailed information about their research question, study design, analysis plan, and potential outcomes before beginning data collection.
What is the purpose of registered reports guidelines for?
The purpose of registered reports guidelines is to promote transparency, reduce publication bias, and ensure that research findings are based on sound scientific principles.
What information must be reported on registered reports guidelines for?
Researchers must report details about their research question, study design, data collection methods, analysis plan, and potential outcomes on registered reports guidelines.
Fill out your registered reports guidelines for online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Registered Reports Guidelines For is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















