Get the free Submission to Medicines Management Committee
Show details
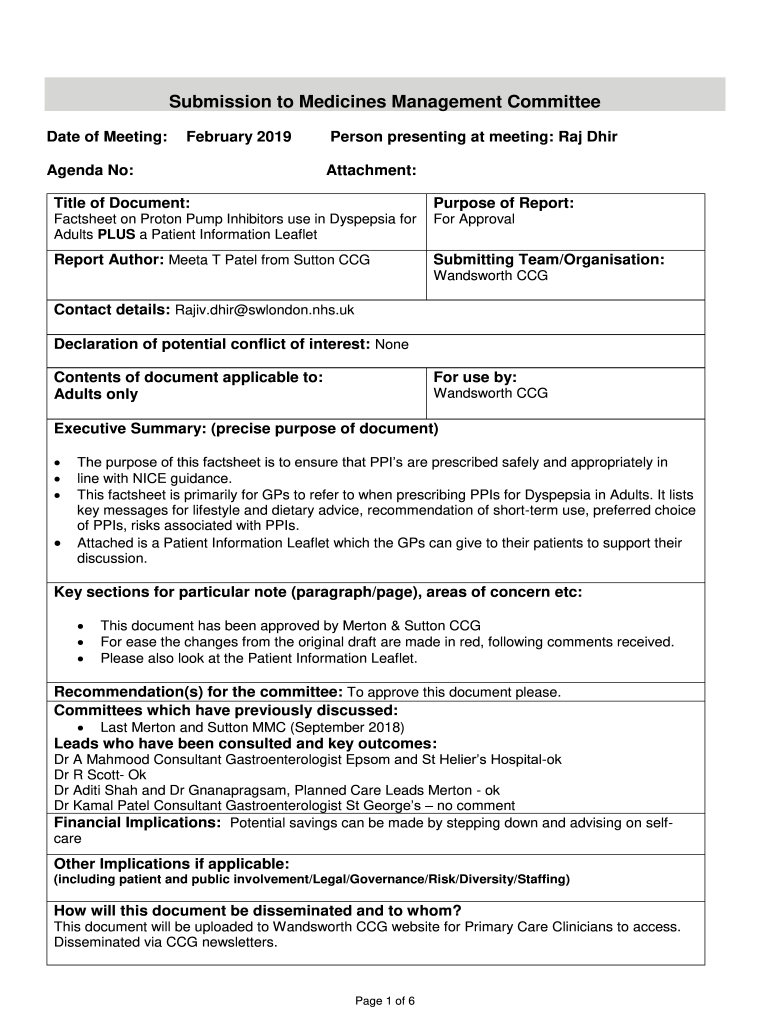
Submission to Medicines Management Committee
Date of Meeting:February 2019Agenda No:Person presenting at meeting: Raj Dir
Attachment:Title of Document:Purpose of Report:Fact sheet on Proton Pump Inhibitors
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign submission to medicines management

Edit your submission to medicines management form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your submission to medicines management form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit submission to medicines management online
To use the services of a skilled PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit submission to medicines management. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out submission to medicines management

How to fill out submission to medicines management
01
To fill out a submission to medicines management, follow these steps:
02
Gather all necessary information about the medicine, such as its name, dosage, and usage instructions.
03
Prepare any supporting documents or evidence that may be required, such as clinical trial results or safety data.
04
Fill in the submission form accurately and completely, providing all requested information.
05
Double-check the form for any errors or omissions before submitting it. Make sure all information is clear and legible.
06
Submit the completed form and supporting documents to the appropriate medicines management authority or organization.
07
Keep a copy of the submission for your records.
08
Await feedback or approval from the medicines management authority. Be prepared to provide additional information or clarify any points if requested.
09
Follow any further instructions or requirements given by the medicines management authority.
10
Monitor the progress of your submission and take necessary actions as instructed.
11
Keep records of any correspondence or communication related to the submission for future reference.
Who needs submission to medicines management?
01
Submission to medicines management is required by healthcare professionals, pharmaceutical companies, researchers, and other relevant stakeholders involved in the development, approval, and monitoring of medicines.
02
Specifically, individuals or organizations seeking to introduce new medicines to the market or make changes to existing medicines often need to submit their proposals or applications to medicines management authorities for evaluation, regulatory compliance, and decision-making purposes.
03
Ultimately, anyone involved in the safe and effective management of medicines may need to undergo the submission process to ensure compliance with regulatory standards and to protect public health.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my submission to medicines management in Gmail?
It's easy to use pdfFiller's Gmail add-on to make and edit your submission to medicines management and any other documents you get right in your email. You can also eSign them. Take a look at the Google Workspace Marketplace and get pdfFiller for Gmail. Get rid of the time-consuming steps and easily manage your documents and eSignatures with the help of an app.
How can I send submission to medicines management for eSignature?
When your submission to medicines management is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
Can I sign the submission to medicines management electronically in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
What is submission to medicines management?
Submission to medicines management refers to the process of formally reporting and documenting medication-related activities, decisions, or changes to ensure proper oversight and compliance with regulatory requirements.
Who is required to file submission to medicines management?
Healthcare professionals, including pharmacists, physicians, and other authorized personnel involved in medication administration and management are typically required to file submissions to medicines management.
How to fill out submission to medicines management?
To fill out a submission to medicines management, you should follow the specified guidelines provided by the regulatory body, including providing accurate patient information, medication details, reason for submission, and any supporting documentation.
What is the purpose of submission to medicines management?
The purpose of submission to medicines management is to ensure patient safety, enhance the quality of medication use, and comply with legal and regulatory standards related to medication management.
What information must be reported on submission to medicines management?
The submission must typically report patient identifiers, medication names, dosages, administration routes, the reason for submission, and any relevant clinical data or observations.
Fill out your submission to medicines management online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Submission To Medicines Management is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















