Get the free Second European evidence-based consensus on the prevention ...
Show details
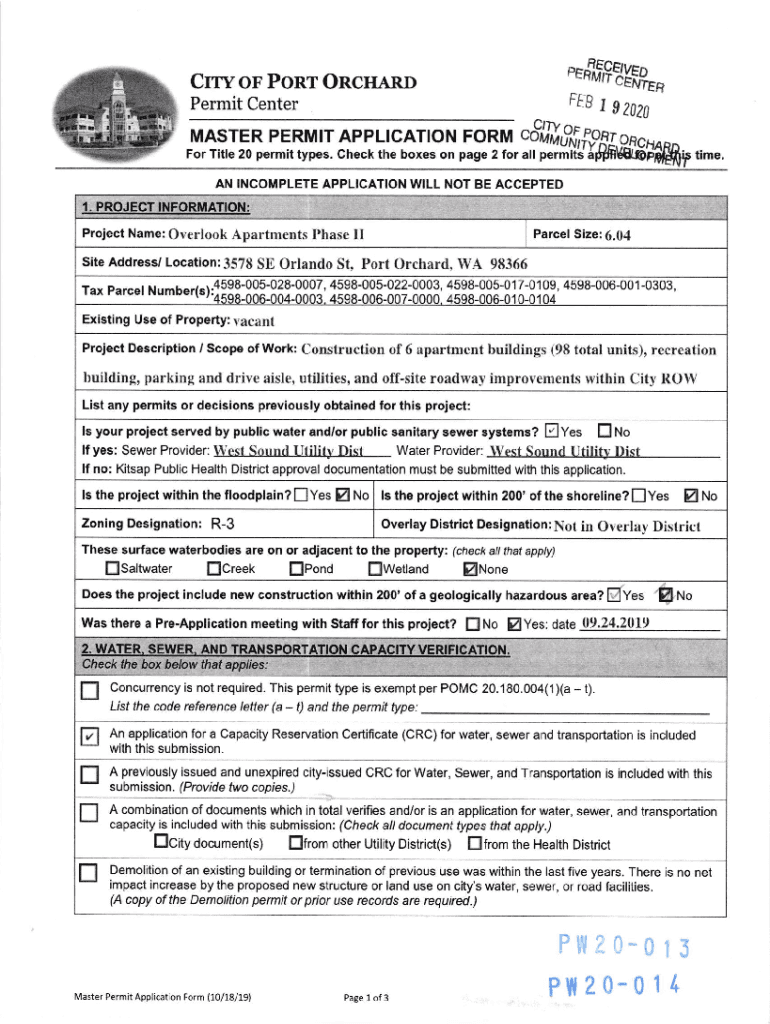
, eflnE9BEi%,CRR or Poor OncnenoFEBPermit CenterMAs1g2020coffi,ffgop1on “For Title 20 permit types. Check the boxes on page 2 for all permit 5pfrld8ll3ffirtime,AN INCOMPLETE APPLICATION WILL NOT
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign second european evidence-based consensus

Edit your second european evidence-based consensus form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your second european evidence-based consensus form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing second european evidence-based consensus online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit second european evidence-based consensus. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out second european evidence-based consensus

How to fill out second european evidence-based consensus
01
To fill out the second European evidence-based consensus, follow these steps:
02
Review the previous European evidence-based consensus.
03
Identify any updates or new information that needs to be included.
04
Gather research evidence, studies, and data to support the consensus.
05
Organize the information into clear sections or points.
06
Write each point or section in a concise and clear manner.
07
Ensure that each point is supported by evidence and references.
08
Review and revise the consensus for accuracy and clarity.
09
Share the draft consensus with experts in the field for feedback.
10
Incorporate any necessary changes based on expert feedback.
11
Finalize the consensus document and publish it for public access.
Who needs second european evidence-based consensus?
01
The second European evidence-based consensus is needed by healthcare professionals, researchers, policymakers, and organizations working in the field of evidence-based medicine.
02
It provides them with a standardized and authoritative document that outlines the current best practices and evidence-based recommendations for specific healthcare topics.
03
This consensus helps guide decision-making, research, and the development of clinical guidelines, ultimately improving patient outcomes and healthcare quality.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send second european evidence-based consensus to be eSigned by others?
Once you are ready to share your second european evidence-based consensus, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
How do I edit second european evidence-based consensus on an iOS device?
No, you can't. With the pdfFiller app for iOS, you can edit, share, and sign second european evidence-based consensus right away. At the Apple Store, you can buy and install it in a matter of seconds. The app is free, but you will need to set up an account if you want to buy a subscription or start a free trial.
How do I fill out second european evidence-based consensus on an Android device?
Use the pdfFiller app for Android to finish your second european evidence-based consensus. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
What is second european evidence-based consensus?
The second European evidence-based consensus refers to a comprehensive agreement or guideline developed by experts in a specific field, aiming to standardize and validate clinical practices across Europe based on the best available evidence.
Who is required to file second european evidence-based consensus?
Healthcare professionals and organizations engaged in clinical research or practice in relevant fields may be required to file the second European evidence-based consensus, particularly those involved in regulatory processes or needing to align with European health standards.
How to fill out second european evidence-based consensus?
To fill out the second European evidence-based consensus, one should follow the specific guidelines provided, including sections on background information, methodology, findings, and recommendations, ensuring that all fields are accurately completed and supported by relevant evidence.
What is the purpose of second european evidence-based consensus?
The purpose of the second European evidence-based consensus is to provide a structured approach to clinical guidelines, improve healthcare quality, ensure safety, and enhance patient outcomes across Europe based on scientific research.
What information must be reported on second european evidence-based consensus?
The information that must be reported includes the consensus statement, evidence supporting the recommendations, methodology used, participant details, and any funding or conflict of interest disclosures.
Fill out your second european evidence-based consensus online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Second European Evidence-Based Consensus is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















