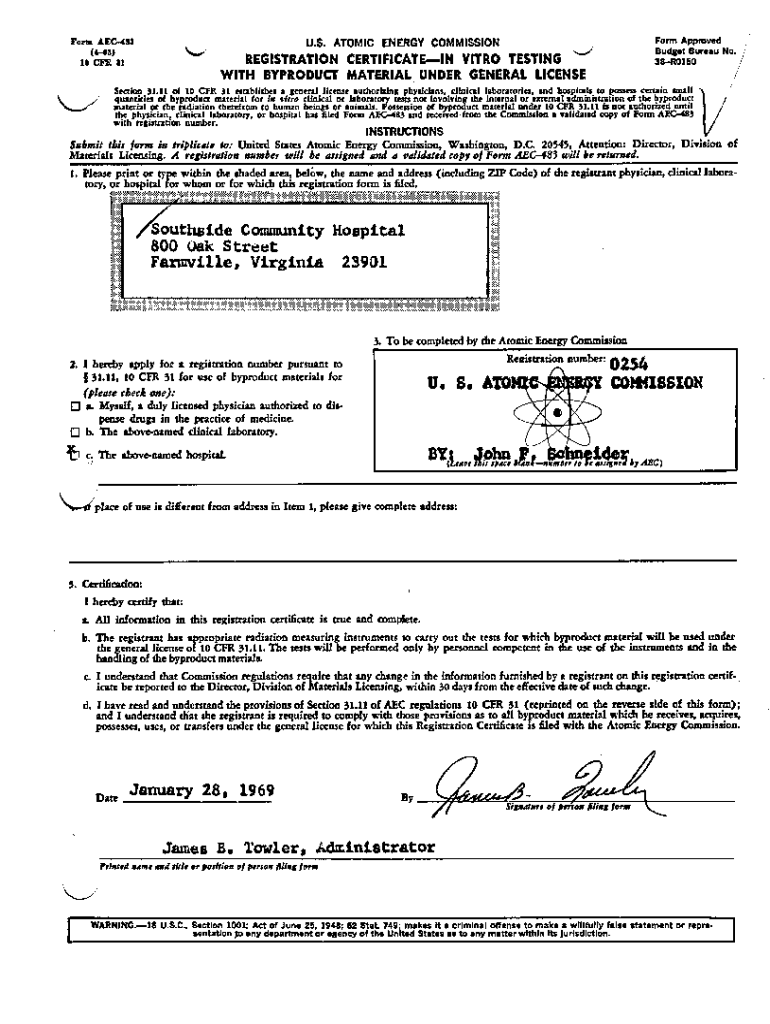
Get the free Registration Certificate for In-Vitro Testing for Southside Community Hospital.
Show details
U.S. ATOMIC ENERGY Commissioner AEC48310(448)Form Approved
Bureau No.\'., Budget REGISTRATION CERTIFICATE IN VITO TESTING
WITH BYPRODUCT MATERIAL UNDER GENERAL LICENSE38RN160Section 31.11 of 10 CFR
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign registration certificate for in-vitro

Edit your registration certificate for in-vitro form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your registration certificate for in-vitro form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing registration certificate for in-vitro online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Sign into your account. It's time to start your free trial.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit registration certificate for in-vitro. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
The use of pdfFiller makes dealing with documents straightforward. Try it now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out registration certificate for in-vitro

How to fill out registration certificate for in-vitro
01
To fill out a registration certificate for in-vitro, follow these steps:
02
Start by filling out the basic information section, including the applicant's name, address, and contact details.
03
Provide details about the in-vitro diagnostic medical device, such as its intended use, classification, and technical specifications.
04
Include information about the manufacturer, such as their name, address, and contact details.
05
Attach relevant supporting documents, such as product technical files, labeling, and a summary of clinical data.
06
Submit the completed registration certificate application along with the necessary fees to the appropriate regulatory authority.
07
Wait for the regulatory authority to review the application and provide feedback or request additional information, if necessary.
08
Once the application is approved, you will receive the registration certificate for your in-vitro diagnostic medical device.
09
Note: The specific requirements and procedures may vary depending on the country or regulatory authority involved. It is essential to consult the relevant guidelines and regulations for accurate and up-to-date information.
Who needs registration certificate for in-vitro?
01
Various stakeholders may require a registration certificate for in-vitro diagnostic medical devices, including:
02
- Manufacturers who intend to market and sell in-vitro diagnostic medical devices.
03
- Importers or distributors who handle in-vitro diagnostic medical devices.
04
- Healthcare facilities, such as hospitals or laboratories, that use in-vitro diagnostic medical devices for patient testing.
05
- Regulatory authorities who assess the safety and performance of in-vitro diagnostic medical devices and ensure compliance with applicable regulations.
06
The exact requirements for obtaining a registration certificate may vary depending on the country or region. It is advisable to consult the specific regulatory authorities or guidelines for accurate and up-to-date information.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit registration certificate for in-vitro online?
With pdfFiller, the editing process is straightforward. Open your registration certificate for in-vitro in the editor, which is highly intuitive and easy to use. There, you’ll be able to blackout, redact, type, and erase text, add images, draw arrows and lines, place sticky notes and text boxes, and much more.
How can I fill out registration certificate for in-vitro on an iOS device?
Install the pdfFiller app on your iOS device to fill out papers. If you have a subscription to the service, create an account or log in to an existing one. After completing the registration process, upload your registration certificate for in-vitro. You may now use pdfFiller's advanced features, such as adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
Can I edit registration certificate for in-vitro on an Android device?
You can. With the pdfFiller Android app, you can edit, sign, and distribute registration certificate for in-vitro from anywhere with an internet connection. Take use of the app's mobile capabilities.
What is registration certificate for in-vitro?
The registration certificate for in-vitro is a document that allows a laboratory or facility to legally operate and perform in-vitro diagnostic tests.
Who is required to file registration certificate for in-vitro?
Any laboratory or facility that performs in-vitro diagnostic tests is required to file a registration certificate for in-vitro.
How to fill out registration certificate for in-vitro?
The registration certificate for in-vitro can usually be filled out online through a designated government website or by completing a paper form and submitting it to the relevant regulatory authority.
What is the purpose of registration certificate for in-vitro?
The purpose of the registration certificate for in-vitro is to ensure that laboratories and facilities conducting in-vitro diagnostic tests meet necessary quality, safety, and performance standards.
What information must be reported on registration certificate for in-vitro?
Typically, the registration certificate for in-vitro requires information such as the laboratory's contact details, accreditation status, details of the in-vitro tests performed, and compliance with relevant regulations.
Fill out your registration certificate for in-vitro online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Registration Certificate For In-Vitro is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















