Get the free Daily Decontamination Procedure Quality Audit - zutron.com
Show details
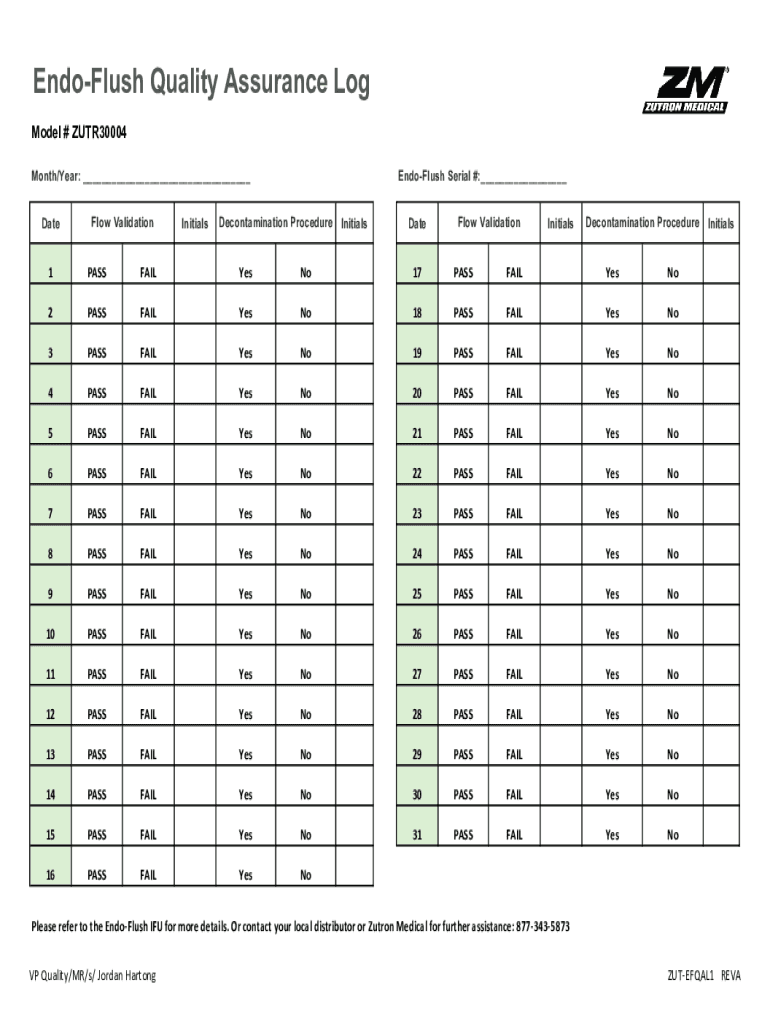
EndoFlush Quality Assurance Log Model # ZUTR30004 Month/Year: Waterloo ValidationInitialsEndoFlush Serial #: Decontamination Procedure InitialsDateFlow ValidationInitialsDecontamination Procedure
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign daily decontamination procedure quality

Edit your daily decontamination procedure quality form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your daily decontamination procedure quality form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing daily decontamination procedure quality online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit daily decontamination procedure quality. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out daily decontamination procedure quality

How to fill out daily decontamination procedure quality
01
Start by gathering all the necessary equipment and materials for the daily decontamination procedure.
02
Put on appropriate personal protective equipment (PPE) such as gloves, goggles, and a lab coat.
03
Begin by thoroughly cleaning and disinfecting all surfaces and equipment that will be used during the decontamination process.
04
Follow the manufacturer's instructions for the cleaning and disinfection products to ensure effective removal of contaminants.
05
Pay special attention to high-touch areas such as doorknobs, light switches, and handles.
06
Use suitable disinfectants or sanitizers to clean the surfaces and materials.
07
Allow sufficient contact time for the disinfectant to effectively eliminate any potential pathogens.
08
Dispose of any used cleaning materials, contaminated waste, and PPE as per the proper disposal guidelines.
09
Regularly monitor and document the decontamination process to ensure compliance with quality standards.
10
Establish a routine schedule for daily decontamination procedures and adhere to it consistently.
11
Regularly review and update the decontamination procedure to incorporate any new guidelines or best practices.
12
Provide appropriate training and education to personnel involved in the daily decontamination process to ensure proper implementation.
13
Continuously assess and improve the decontamination procedure to enhance quality and effectiveness.
Who needs daily decontamination procedure quality?
01
Anyone who works in an environment where there is a risk of contamination or the presence of pathogens needs to follow the daily decontamination procedure quality.
02
This includes healthcare facilities, laboratories, food processing plants, research facilities, and any other setting where cleanliness and hygiene are crucial.
03
Following the daily decontamination procedure quality helps to maintain a safe and sanitary environment, prevent the spread of infectious diseases, and ensure the well-being of both employees and individuals who interact with the facility.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the daily decontamination procedure quality in Chrome?
Yes. By adding the solution to your Chrome browser, you can use pdfFiller to eSign documents and enjoy all of the features of the PDF editor in one place. Use the extension to create a legally-binding eSignature by drawing it, typing it, or uploading a picture of your handwritten signature. Whatever you choose, you will be able to eSign your daily decontamination procedure quality in seconds.
Can I create an electronic signature for signing my daily decontamination procedure quality in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your daily decontamination procedure quality directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
Can I edit daily decontamination procedure quality on an Android device?
The pdfFiller app for Android allows you to edit PDF files like daily decontamination procedure quality. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is daily decontamination procedure quality?
Daily decontamination procedure quality refers to the systematic and standardized processes implemented to ensure that all contaminated areas and equipment are thoroughly cleaned and disinfected on a daily basis, to maintain safety and hygiene standards.
Who is required to file daily decontamination procedure quality?
Individuals or teams responsible for maintaining cleanliness in facilities where contamination is a risk, such as healthcare workers, sanitation staff, and facility managers, are typically required to file the daily decontamination procedure quality.
How to fill out daily decontamination procedure quality?
To fill out the daily decontamination procedure quality, one should document the date, time, area decontaminated, methods used, materials employed, personnel involved, and any observations or issues encountered during the process.
What is the purpose of daily decontamination procedure quality?
The purpose of the daily decontamination procedure quality is to reduce the risk of infection, ensure a safe environment for occupants, comply with regulatory standards, and maintain operational integrity.
What information must be reported on daily decontamination procedure quality?
Information to be reported includes the date of decontamination, specific areas cleaned, cleaning materials used, personnel involved, any incidents noted, and the outcome of the decontamination process.
Fill out your daily decontamination procedure quality online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Daily Decontamination Procedure Quality is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















