Get the free Interim Guidelines for Clinical Specimens for COVID-19CDCInterim Guidelines for Clin...
Show details
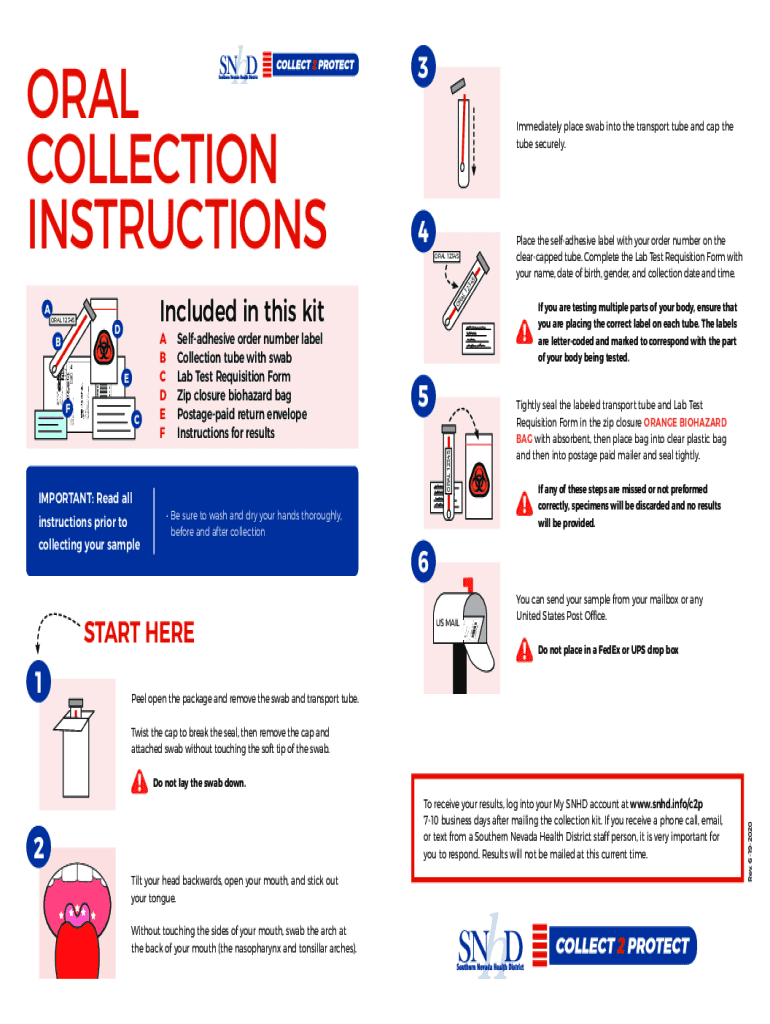
ORAL COLLECTION INSTRUCTIONS ORAL 12345B4ORAL 12345FCIMPORTANT: Read all instructions prior to collecting your sampleABCDEFSelfadhesive order number label Collection tube with swab Lab Test Requisition
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign interim guidelines for clinical

Edit your interim guidelines for clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your interim guidelines for clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing interim guidelines for clinical online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit interim guidelines for clinical. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out interim guidelines for clinical

How to fill out interim guidelines for clinical
01
Step 1: Review the current clinical guidelines and any relevant research or evidence-based practices.
02
Step 2: Identify the specific areas or topics that require interim guidelines due to emerging or time-sensitive information.
03
Step 3: Determine the format and structure of the interim guidelines, considering clarity, accessibility, and ease of use by healthcare professionals.
04
Step 4: Clearly state the purpose and scope of the interim guidelines, including the intended audience and any limitations.
05
Step 5: Gather a multidisciplinary team of experts to draft the interim guidelines, ensuring representation from relevant specialties or subspecialties.
06
Step 6: Conduct a thorough literature review and evidence synthesis to support the recommendations within the interim guidelines.
07
Step 7: Clearly outline the recommendations using a standardized format, including the level of evidence and strength of the recommendation for each.
08
Step 8: Consider potential conflicts of interest within the expert team and disclose any relevant financial or professional relationships.
09
Step 9: Provide clear instructions on how to implement the interim guidelines and any necessary monitoring or follow-up procedures.
10
Step 10: Review and revise the interim guidelines based on feedback and new evidence as it becomes available.
11
Step 11: Obtain approval from relevant regulatory bodies or professional organizations before finalizing and disseminating the interim guidelines.
12
Step 12: Regularly update and communicate any changes or updates to the interim guidelines as new information or evidence emerges.
Who needs interim guidelines for clinical?
01
Clinicians and healthcare professionals who are involved in providing clinical care or making healthcare decisions based on up-to-date evidence.
02
Medical researchers and academics who require guidance on interim practices while conducting clinical studies or trials.
03
Healthcare organizations, hospitals, and medical institutions responsible for developing protocols and policies for clinical care during evolving situations.
04
Government agencies and regulatory bodies that oversee and regulate clinical practices to ensure patient safety and quality of care.
05
Professional organizations and medical societies that aim to provide guidance and standardize clinical practices across their respective specialties.
06
Healthcare policymakers and administrators who need evidence-based recommendations to inform resource allocation and healthcare planning.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send interim guidelines for clinical to be eSigned by others?
Once your interim guidelines for clinical is complete, you can securely share it with recipients and gather eSignatures with pdfFiller in just a few clicks. You may transmit a PDF by email, text message, fax, USPS mail, or online notarization directly from your account. Make an account right now and give it a go.
How can I get interim guidelines for clinical?
It's simple with pdfFiller, a full online document management tool. Access our huge online form collection (over 25M fillable forms are accessible) and find the interim guidelines for clinical in seconds. Open it immediately and begin modifying it with powerful editing options.
How do I fill out interim guidelines for clinical using my mobile device?
On your mobile device, use the pdfFiller mobile app to complete and sign interim guidelines for clinical. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
What is interim guidelines for clinical?
Interim guidelines for clinical are temporary protocols and procedures that outline the necessary steps to conduct clinical trials in a safe and ethical manner.
Who is required to file interim guidelines for clinical?
All companies and research institutions conducting clinical trials are required to file interim guidelines for clinical.
How to fill out interim guidelines for clinical?
Interim guidelines for clinical can be filled out by following the instructions provided by the regulatory authorities and ensuring all required information is accurately documented.
What is the purpose of interim guidelines for clinical?
The purpose of interim guidelines for clinical is to ensure that clinical trials are conducted in compliance with regulations, to protect the safety and welfare of participants, and to produce reliable data for the evaluation of product efficacy and safety.
What information must be reported on interim guidelines for clinical?
Interim guidelines for clinical must include information on study design, participant eligibility criteria, investigational product details, study procedures, adverse event reporting, and data handling.
Fill out your interim guidelines for clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Interim Guidelines For Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















