Get the free Regulatory Research Committee
Show details
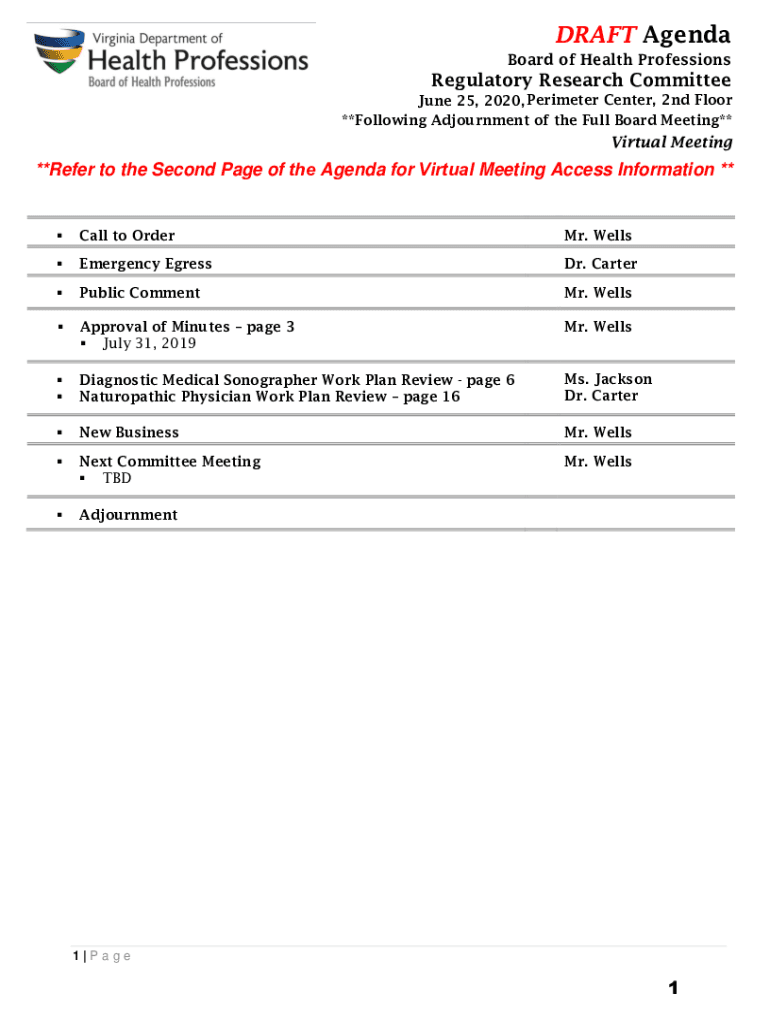
DRAFT Agenda Board of Health ProfessionsRegulatory Research Committee June 25, 2020, Perimeter Center, 2nd Floor **Following Adjournment of the Full Board Meeting**Virtual Meeting**Refer to the Second
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign regulatory research committee

Edit your regulatory research committee form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your regulatory research committee form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing regulatory research committee online
Follow the guidelines below to use a professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit regulatory research committee. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out regulatory research committee

How to fill out regulatory research committee
01
To fill out a regulatory research committee, follow these steps:
02
Review the regulatory requirements and guidelines set by the relevant authorities
03
Identify the key areas that need to be researched and addressed
04
Form a team or committee consisting of knowledgeable individuals in the field
05
Assign specific tasks and responsibilities to each member of the committee
06
Conduct thorough research on the regulatory landscape, including any new updates or changes
07
Evaluate the impact of the regulations on the organization or industry
08
Gather and analyze data, including statistical information, to support the research findings
09
Develop strategies and recommendations to ensure compliance with the regulations
10
Document the research findings, methodologies, and conclusions
11
Present the findings and recommendations to the relevant stakeholders or decision-makers
12
Update the regulatory research committee as needed to address any new regulations or changes.
Who needs regulatory research committee?
01
A regulatory research committee is typically needed by organizations or industries that are subject to regulatory requirements and oversight.
02
This includes but is not limited to:
03
- Government agencies
04
- Financial institutions
05
- Healthcare providers
06
- Pharmaceutical companies
07
- Energy and utilities companies
08
- Manufacturing companies
09
- Environmental organizations
10
- Compliance departments of various businesses.
11
Any organization or industry that needs to ensure compliance with regulations and stay updated with the latest developments can benefit from having a regulatory research committee.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send regulatory research committee for eSignature?
When you're ready to share your regulatory research committee, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
Can I sign the regulatory research committee electronically in Chrome?
Yes. With pdfFiller for Chrome, you can eSign documents and utilize the PDF editor all in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a handwritten signature image. You may eSign your regulatory research committee in seconds.
How do I complete regulatory research committee on an Android device?
On an Android device, use the pdfFiller mobile app to finish your regulatory research committee. The program allows you to execute all necessary document management operations, such as adding, editing, and removing text, signing, annotating, and more. You only need a smartphone and an internet connection.
What is regulatory research committee?
A regulatory research committee is a group established to oversee and ensure compliance with regulatory requirements related to research activities, often involving the review of research proposals to ensure they meet ethical and legal standards.
Who is required to file regulatory research committee?
Researchers conducting studies that involve human subjects, animals, or sensitive data are typically required to file with the regulatory research committee to ensure that their research adheres to ethical guidelines and regulations.
How to fill out regulatory research committee?
To fill out the regulatory research committee form, you typically need to provide detailed information about the research project, including objectives, methodologies, data management plans, and compliance with ethical standards.
What is the purpose of regulatory research committee?
The purpose of the regulatory research committee is to protect the rights and welfare of research participants, ensure that research complies with ethical standards, and minimize risks associated with research activities.
What information must be reported on regulatory research committee?
Information that must be reported includes study title, principal investigator details, research objectives, funding sources, participant recruitment methods, consent procedures, data handling, and potential risks.
Fill out your regulatory research committee online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Regulatory Research Committee is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















